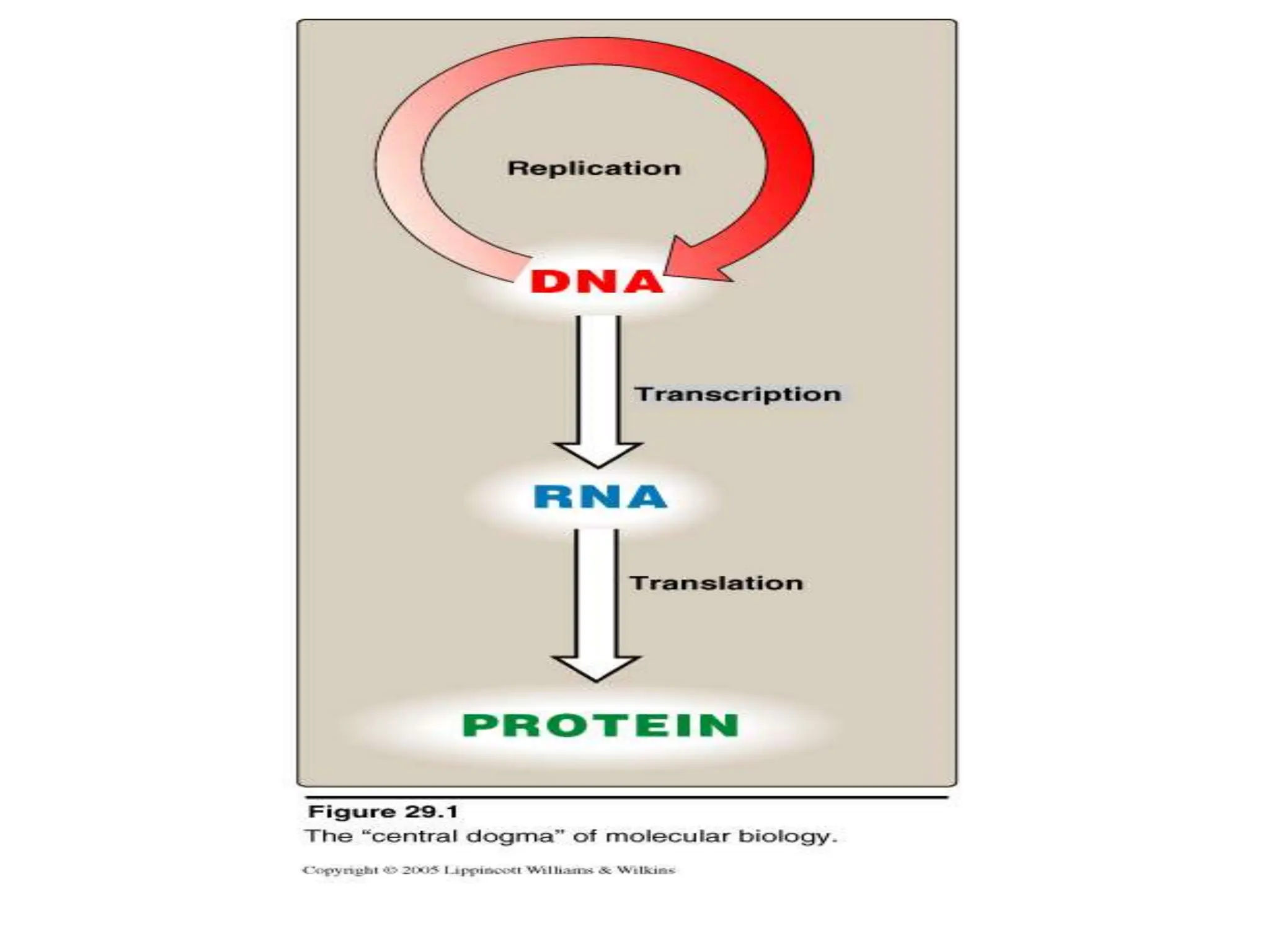
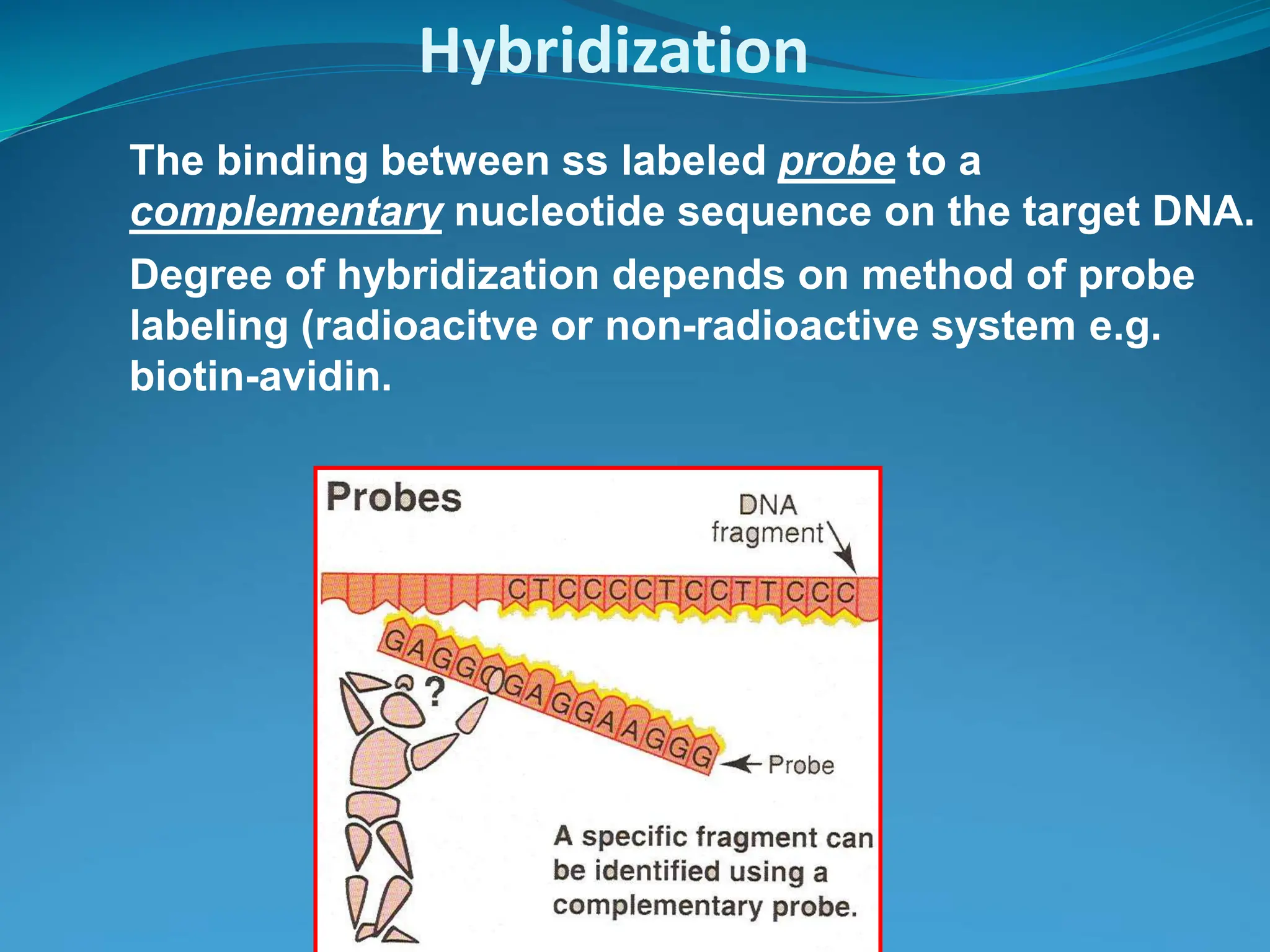
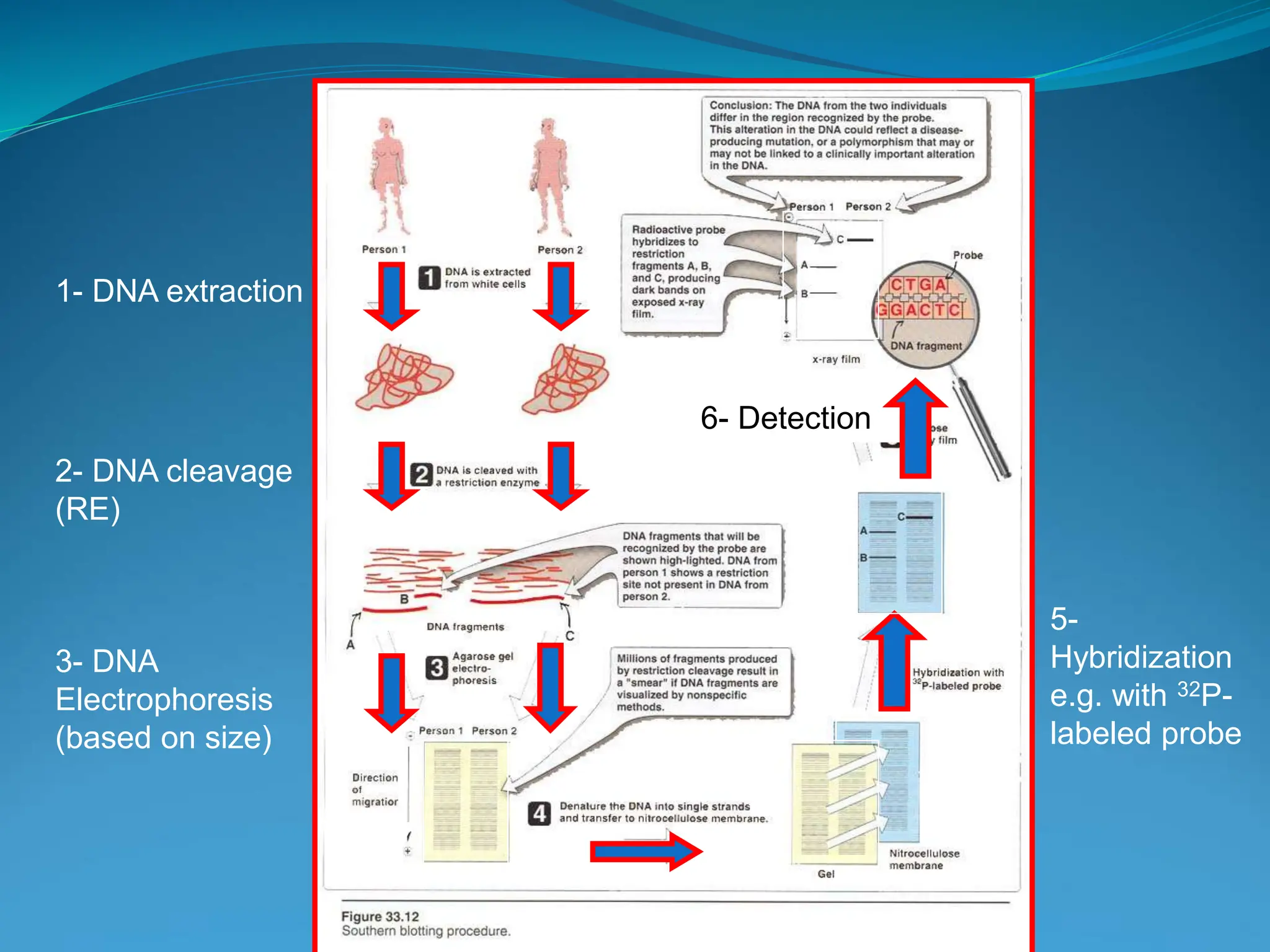
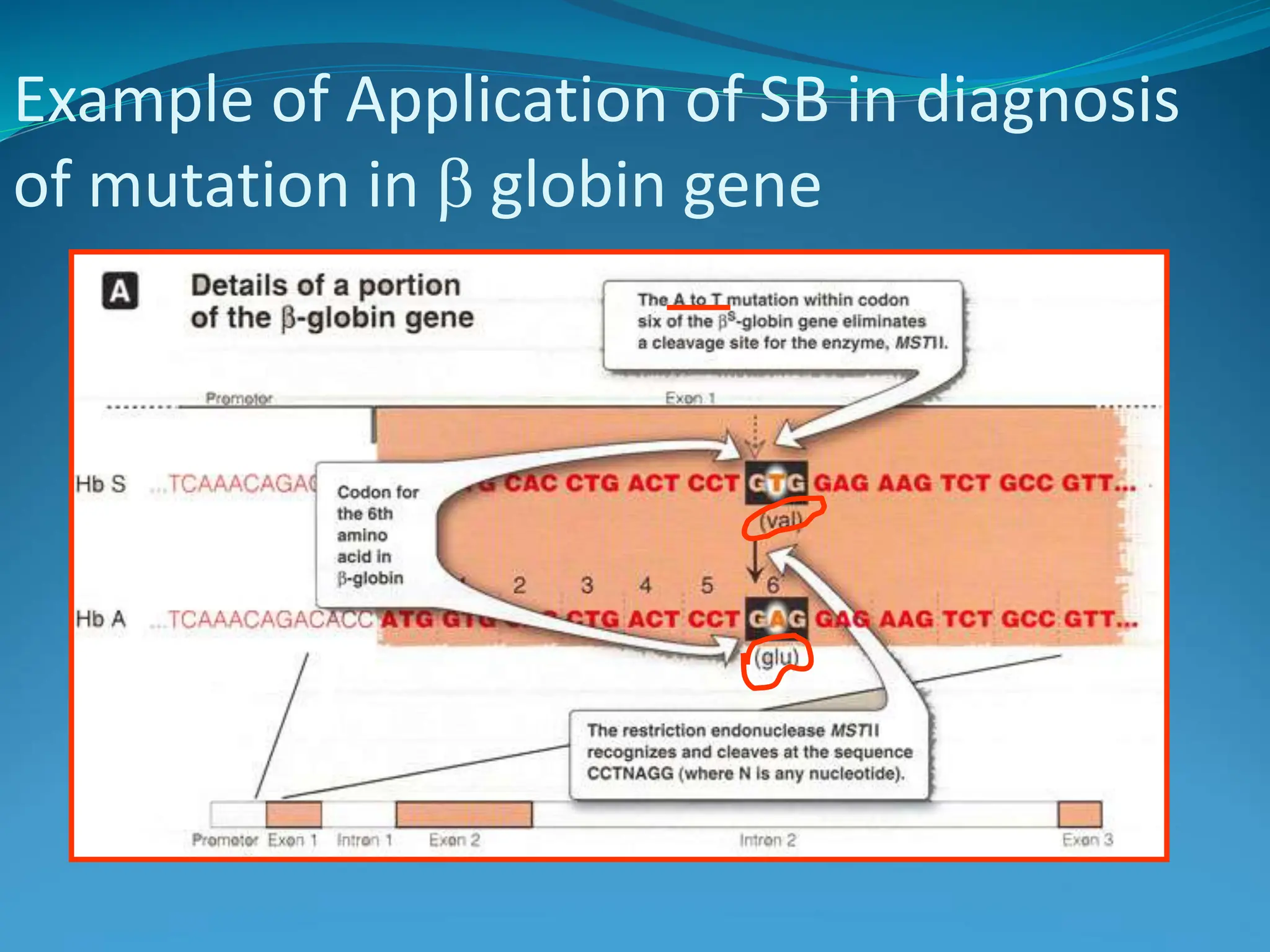
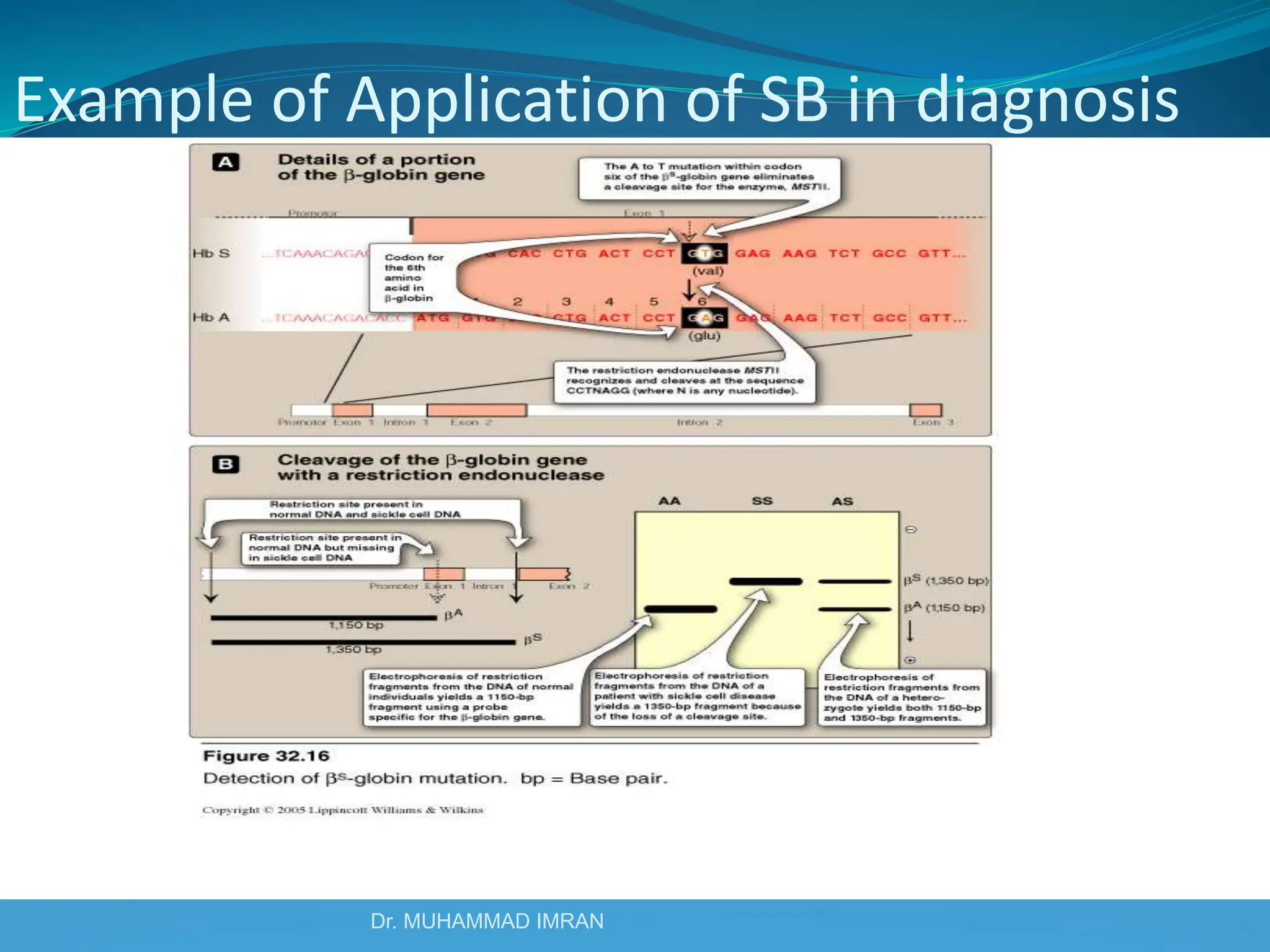
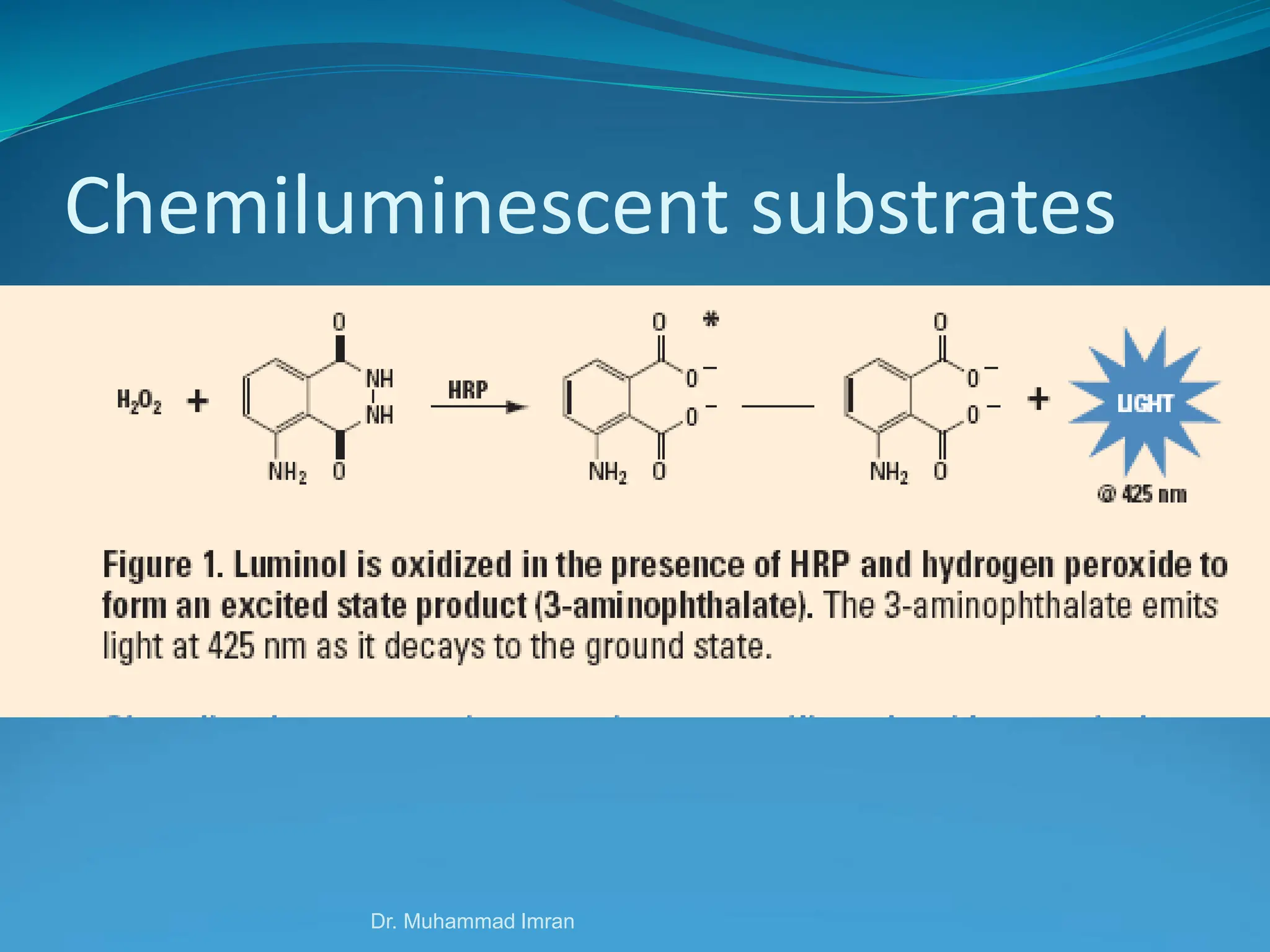
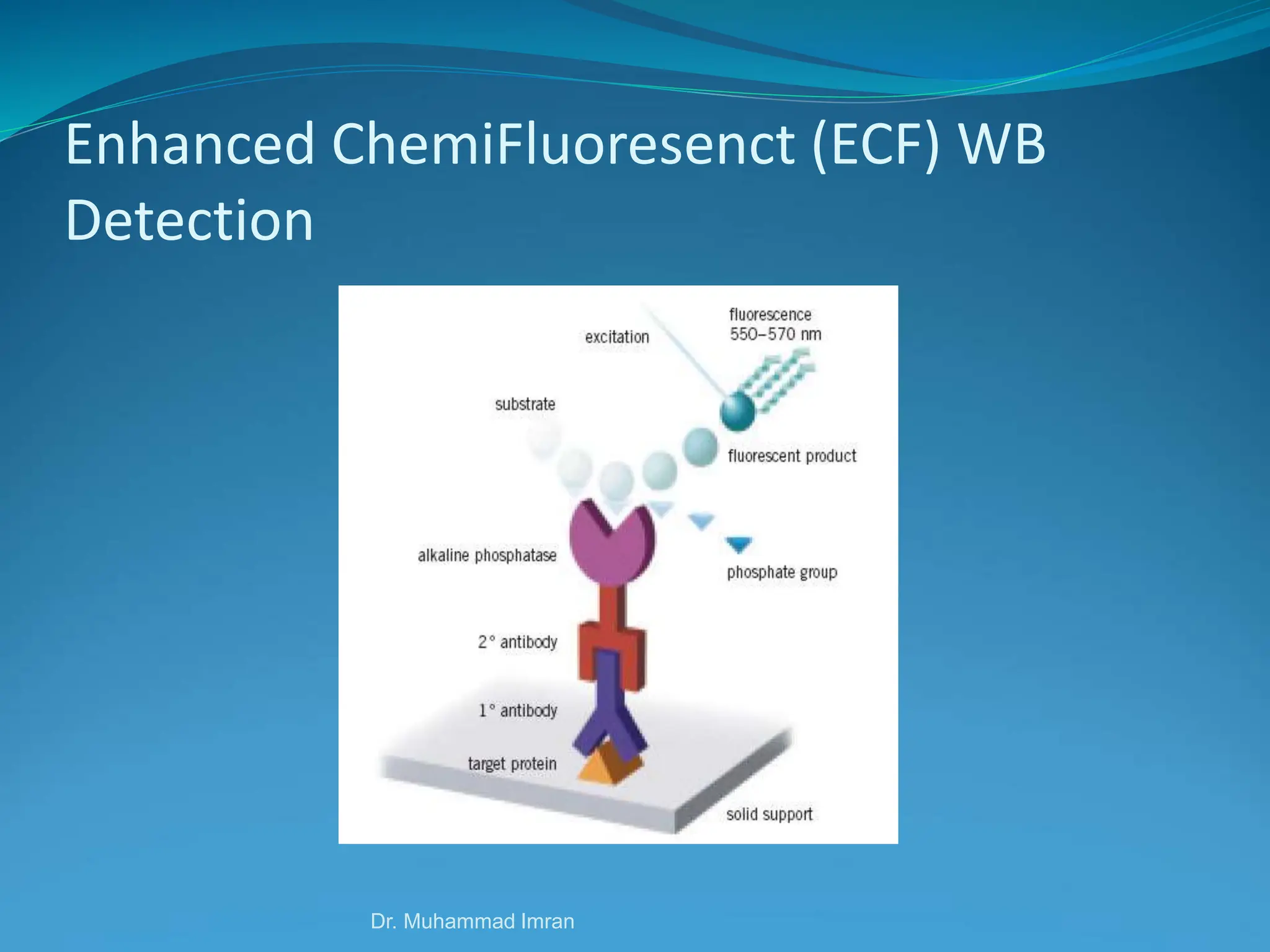
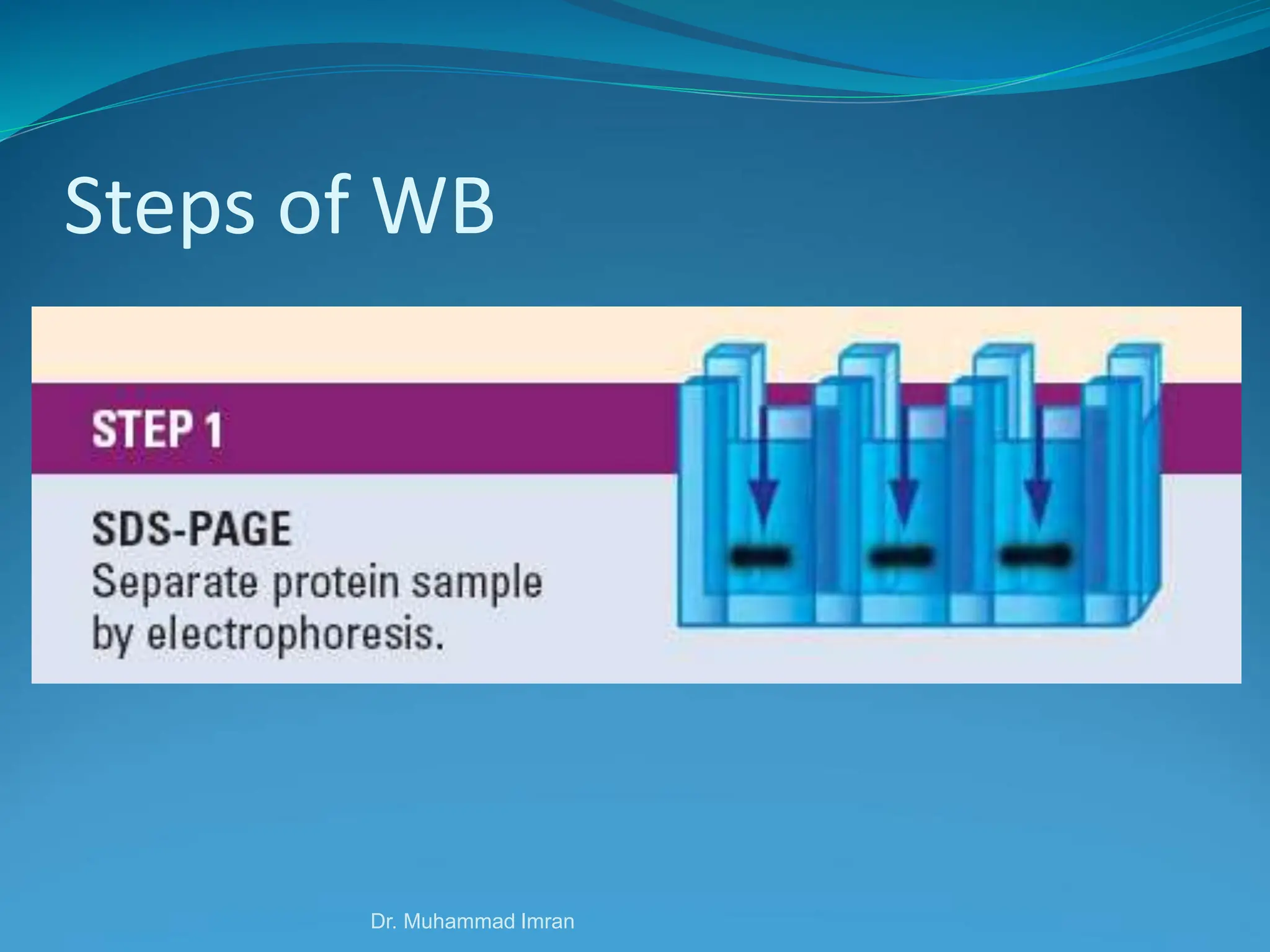
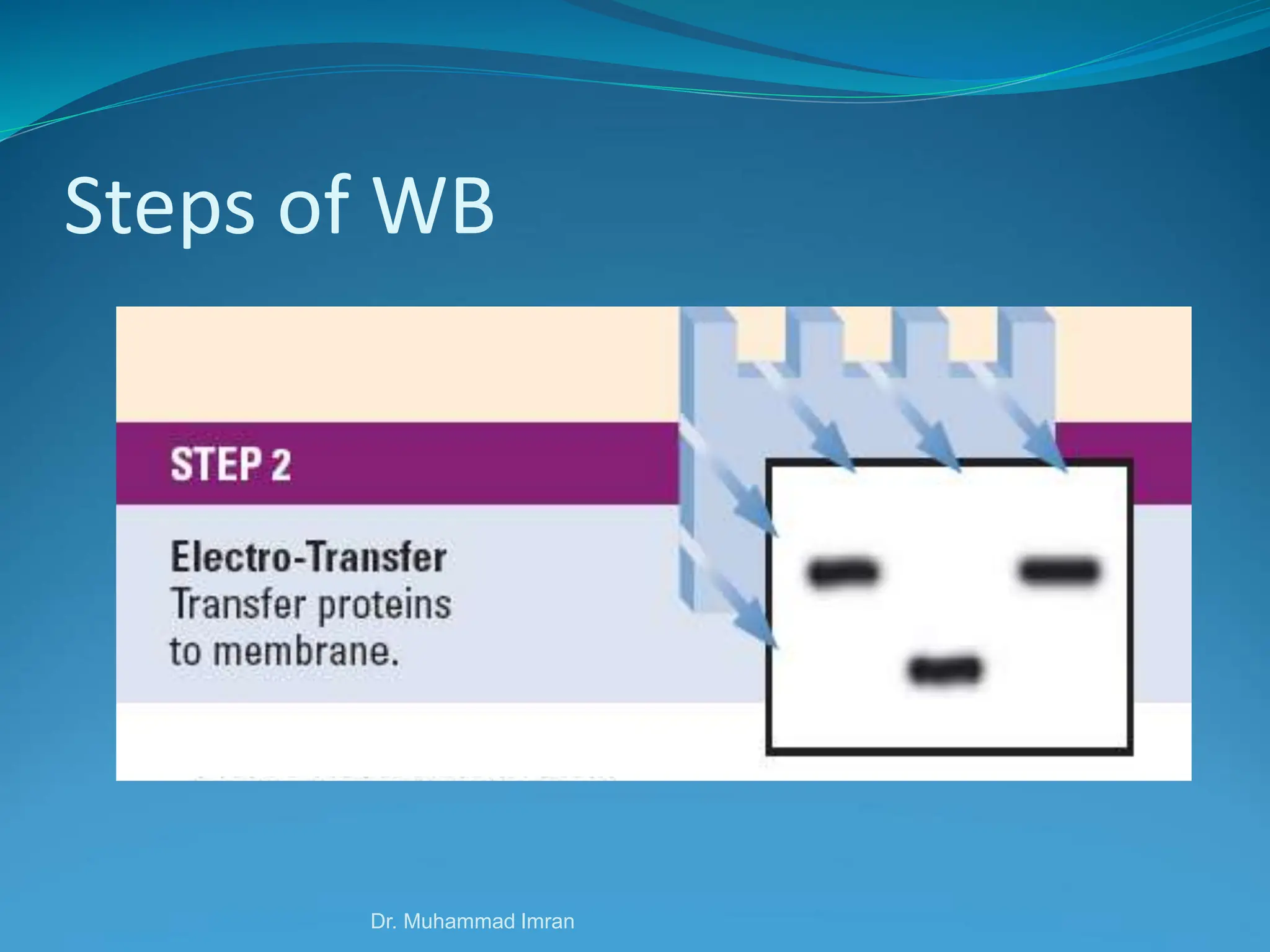
This document outlines various blotting techniques, including Southern, Northern, and Western blotting, detailing their history, main applications, and procedures. Key objectives include understanding the concepts, advantages, and methods of these techniques, as well as their use in diagnosing diseases. The document also highlights the similarities and differences between these methods and discusses specific steps and examples in the context of molecular biology.