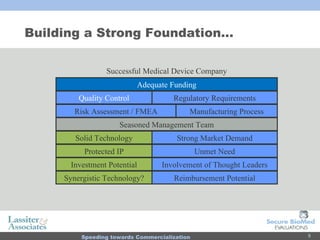
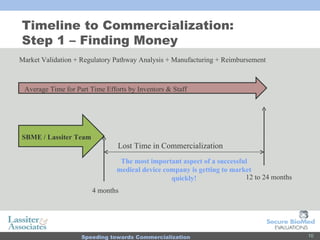
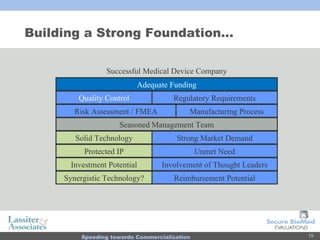
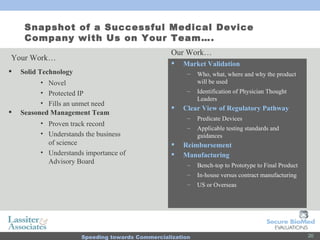
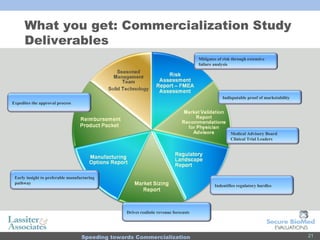
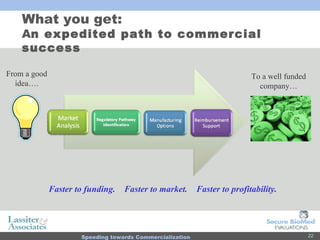
The document discusses commercialization services for emerging biotechnology developments and medical devices. It introduces Secure BioMed Evaluations and Lassiter & Associates, who have expertise in bringing medical devices to market. Their services include evaluating commercial feasibility, regulatory pathway analysis, manufacturing analysis, reimbursement support, market validation, and market sizing to help clients expedite the process of getting products to market.