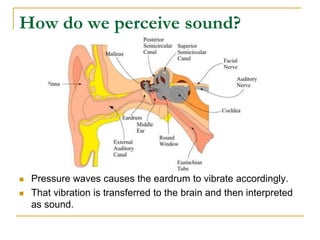
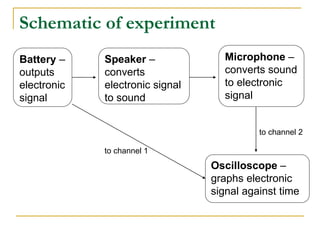
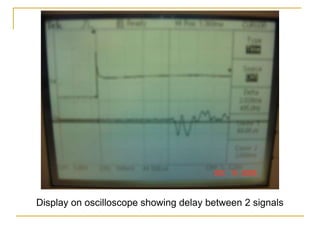
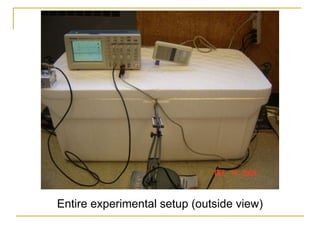
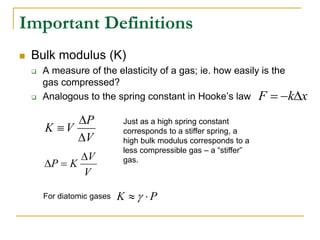
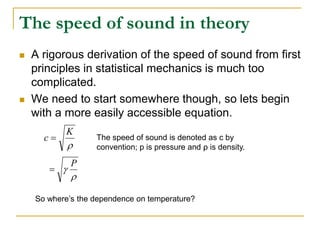
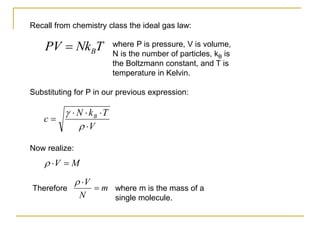
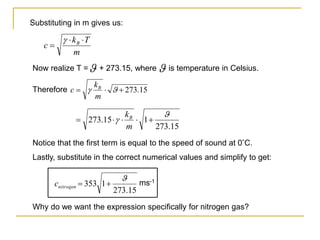
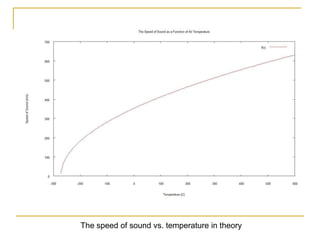
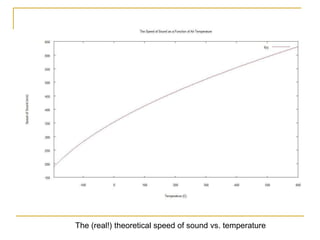
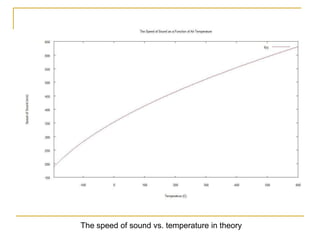
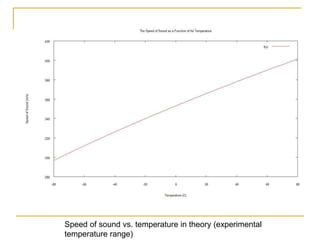
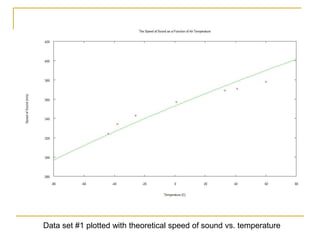
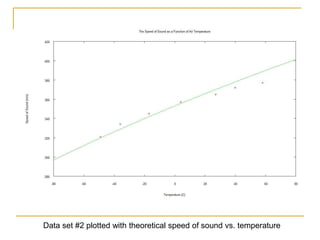
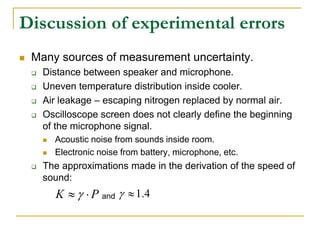
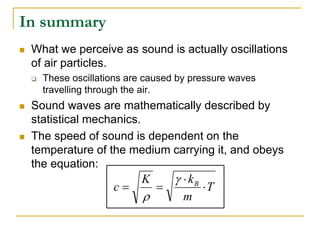
This document describes an experiment to investigate how the speed of sound varies with temperature in air. The experiment involves using a speaker and microphone to measure the time delay of a sound wave traveling through air at different temperatures, controlled by cooling with liquid nitrogen and heating with lamps. The results are analyzed and found to closely match the theoretical prediction from statistical mechanics that the speed of sound increases with temperature according to the given equation. Sources of experimental error are also discussed.