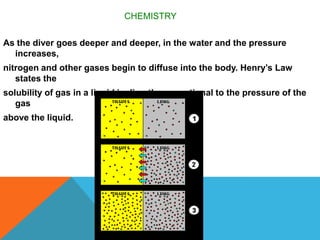
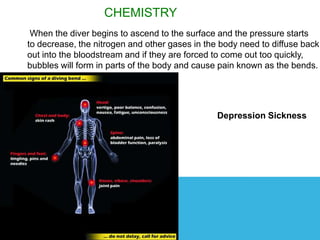
The document discusses the history and science of scuba diving. It explains that early divers held their breath underwater but that compressed air tanks were later developed allowing divers to breathe underwater. It also describes how nitrogen and other gases can cause decompression sickness if a diver ascends to the surface too quickly due to changing water pressure, and that divers must carefully manage their ascent to avoid this.