Dr. Nazuk Sharma presented on placebos. Some key points:
1. A placebo is a dummy medicine containing no active substance that can produce physiological or psychological effects through a patient's expectations.
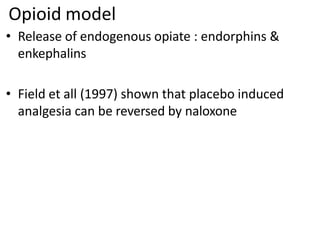
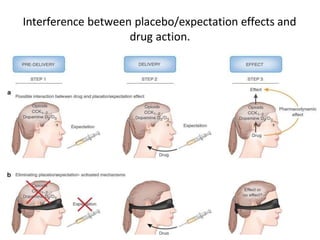
2. Placebos have been used since the 18th century and the placebo effect is thought to be mediated by factors like conditioning, expectations, and the endogenous opioid system.
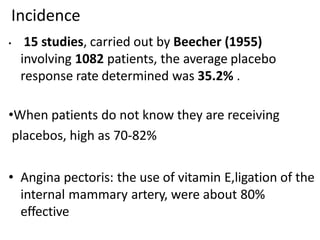
3. Placebo response rates in clinical trials average around 35% but can be as high as 70-80% when patients don't know they are receiving placebos. Placebos are used in clinical trials to compare to active treatments and identify placebo responders.