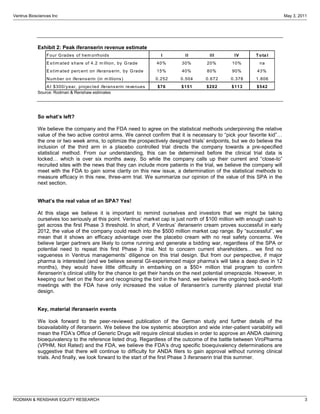
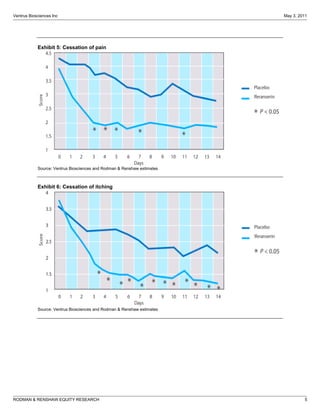
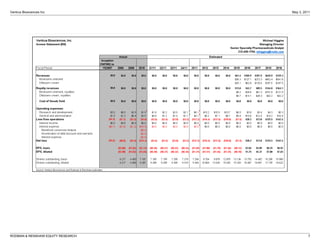
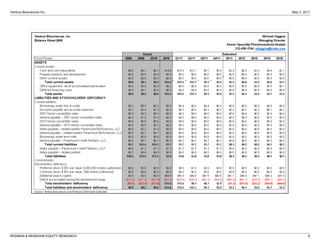
The FDA has proposed two changes to Ventrus Biosciences' Phase 3 trial design for their drug iferanserin cream for hemorrhoids. First, they proposed modifying the primary endpoint. Second, they now propose adding a third treatment arm involving application of the cream for 7 days followed by a placebo for 7 days. This reduces the overall treatment duration. The analyst believes these changes could improve the likelihood of success in the trials and increase the commercial potential of iferanserin cream by making it suitable for shorter-term treatment of less severe hemorrhoid cases. They therefore increase their price target for Ventrus stock from $12 to $25 per share.