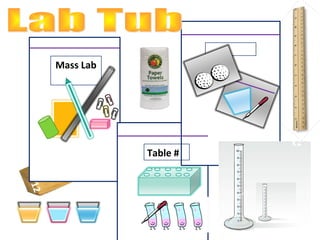
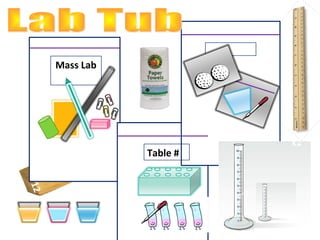
1. The document provides instructions for a science class, listing the materials needed for labs on volume and mass.
2. Students are reminded to staple lab worksheets in order and submit progress notices and log updates.
3. Cleaning instructions are provided to leave the lab space tidy.