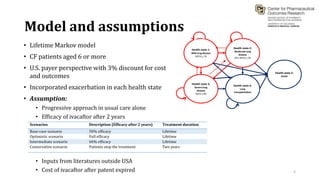
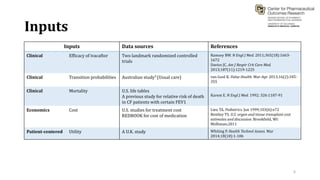
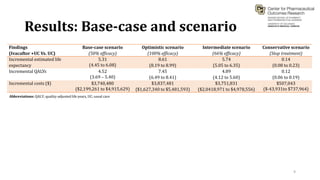
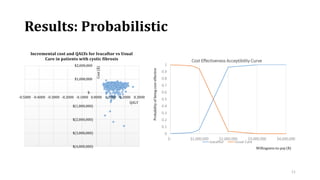
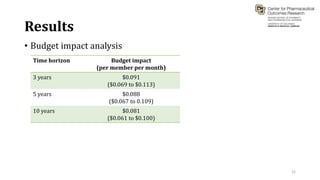
This document summarizes a workshop on cost-effectiveness analysis for respiratory health technologies. The workshop objectives were to introduce cost-effectiveness modeling, discuss what can and cannot be done with these models, and review current evidence gaps. The document then summarizes a cost-effectiveness model developed for ivacaftor treatment of cystic fibrosis. The model found ivacaftor to be cost-effective compared to usual care. Key gaps in COPD and asthma cost-effectiveness studies were identified. Finally, forming a working group to address these evidence gaps through additional research was discussed.