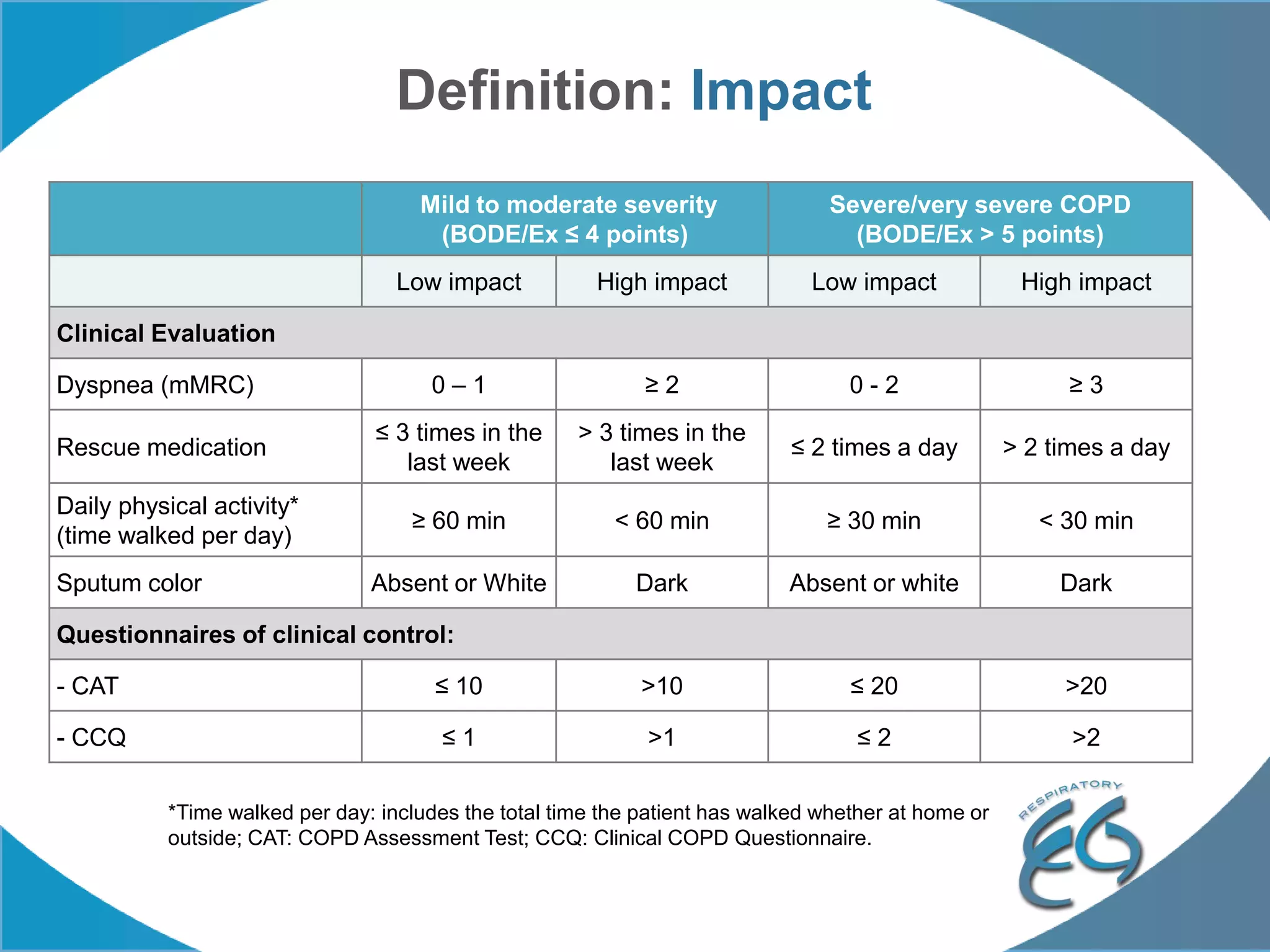
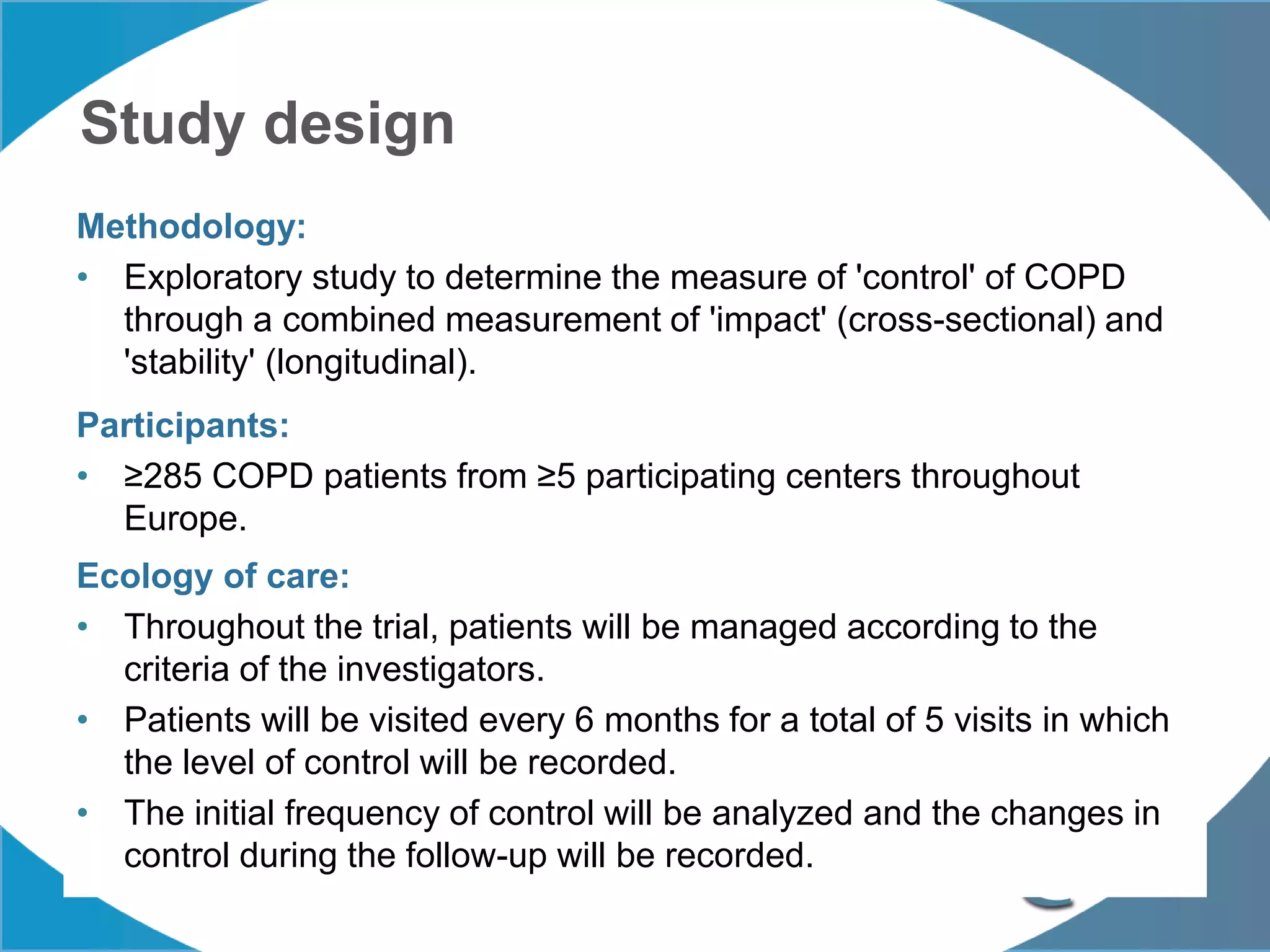
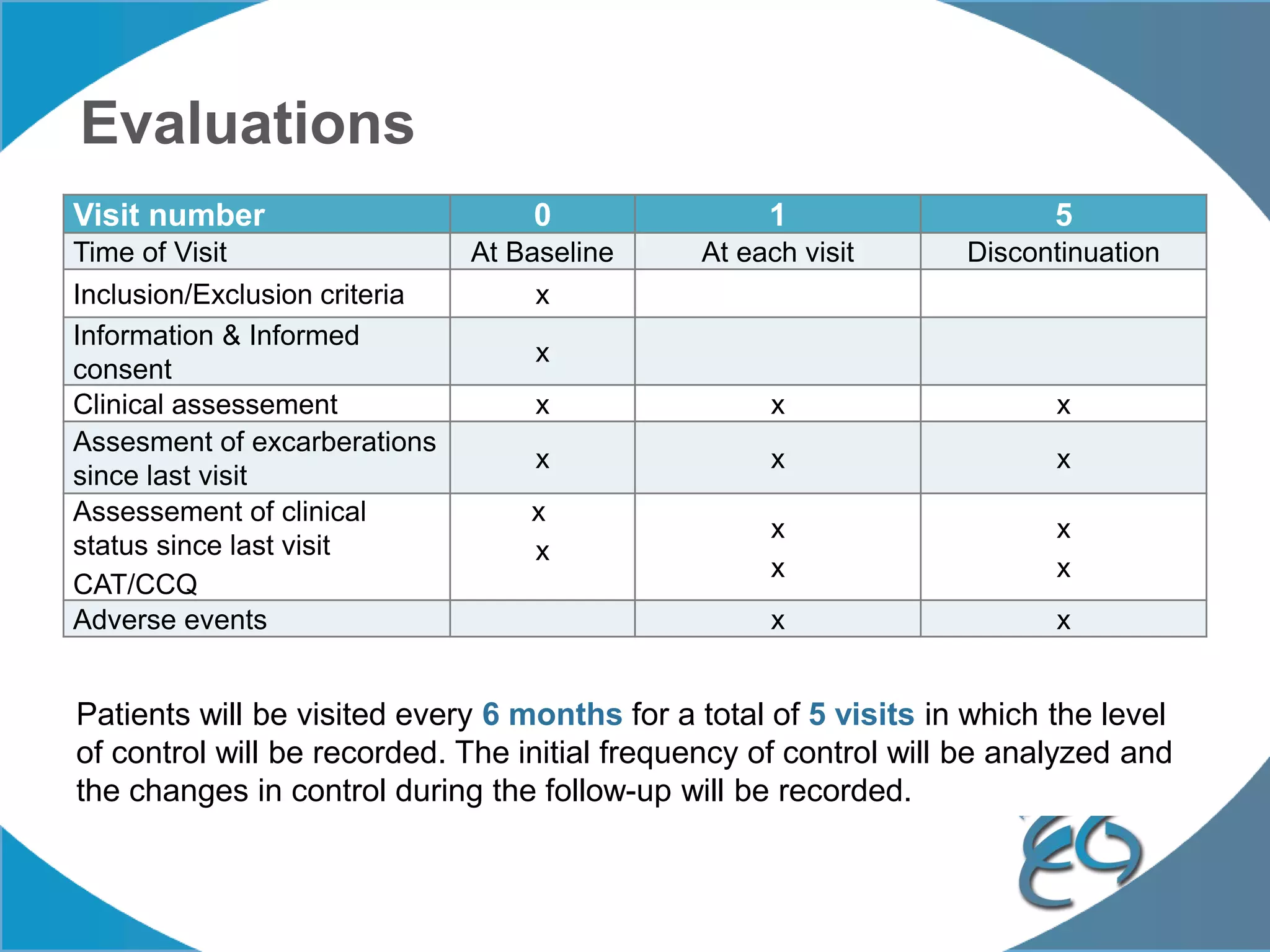
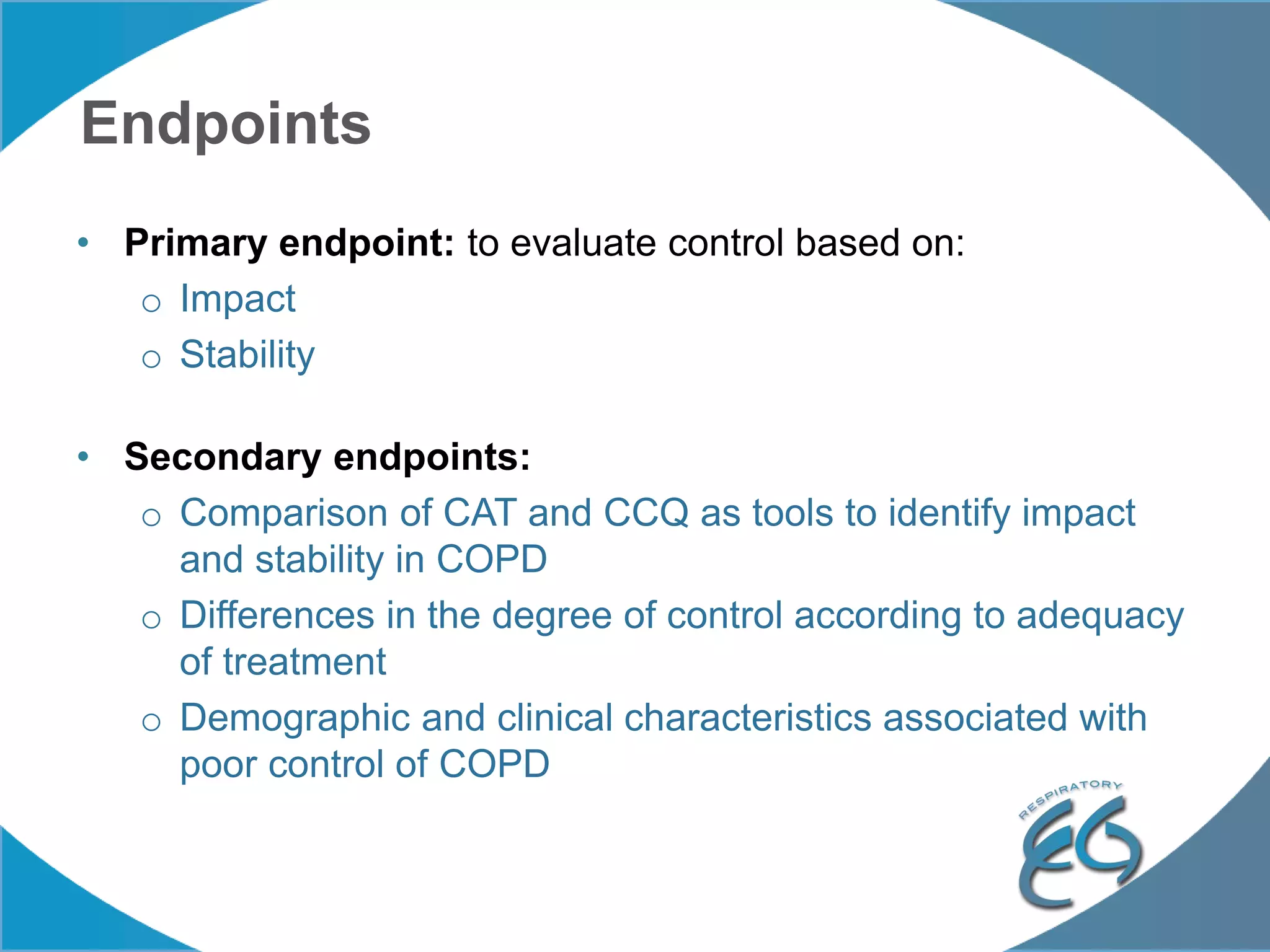
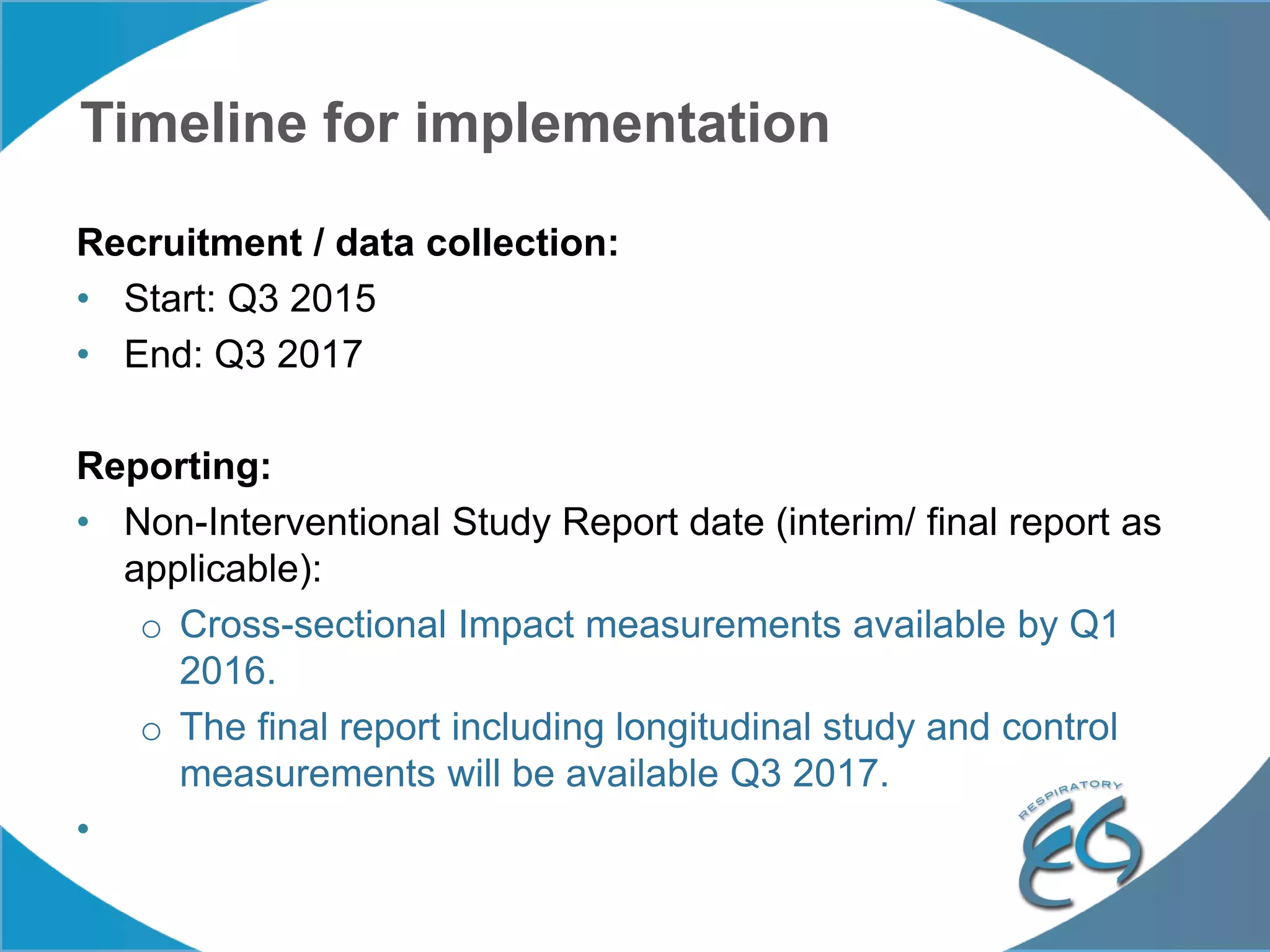
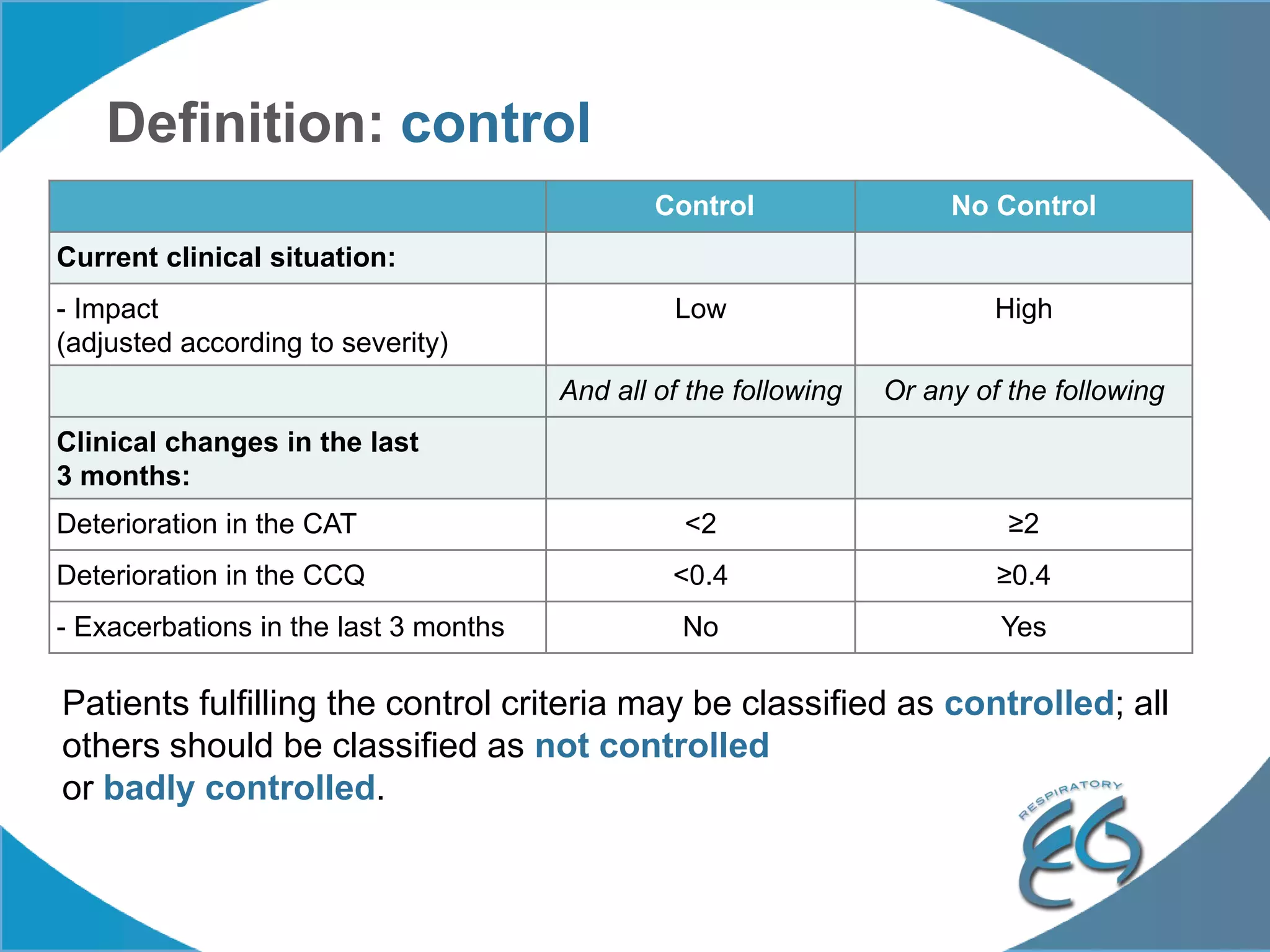
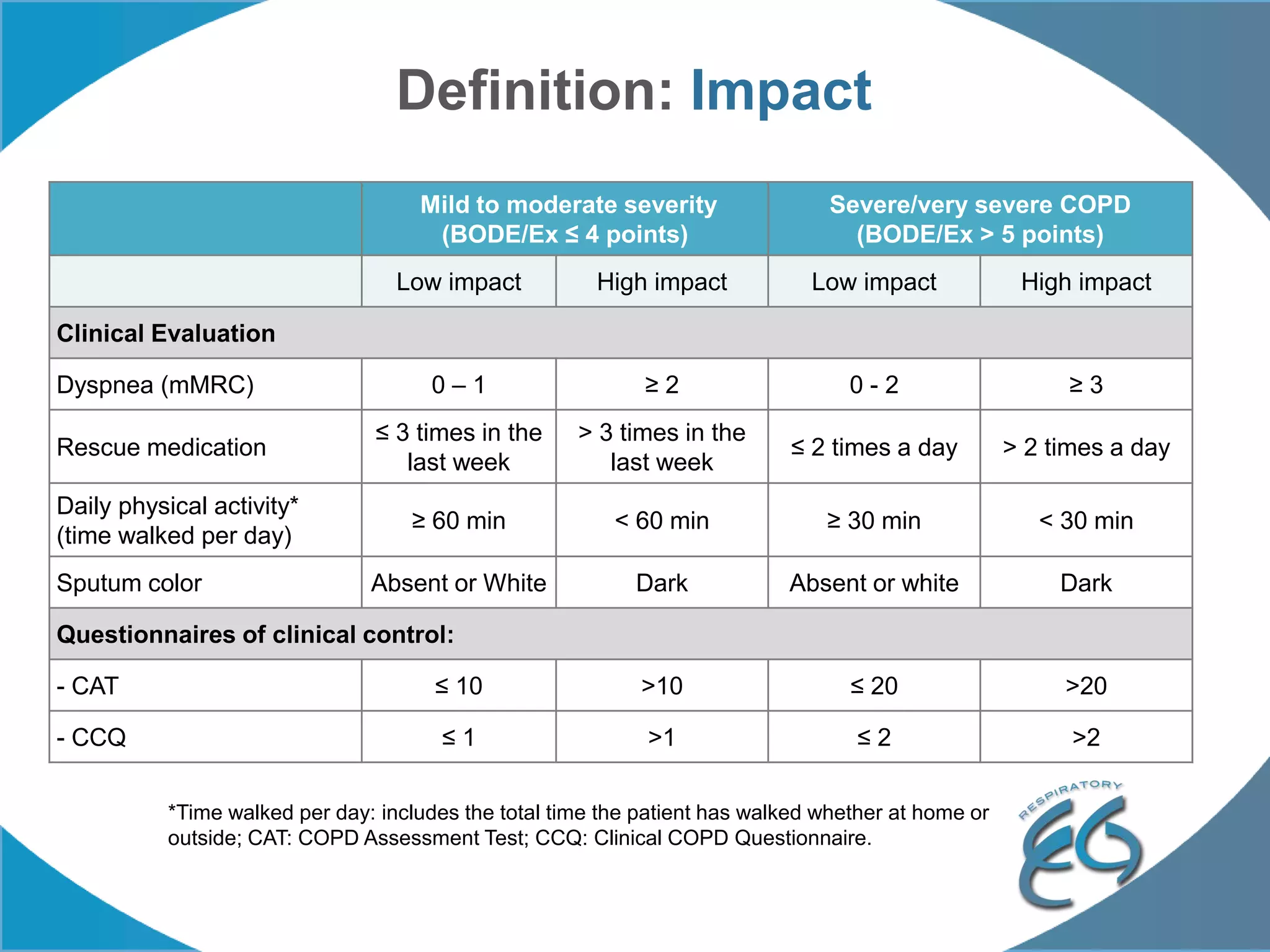
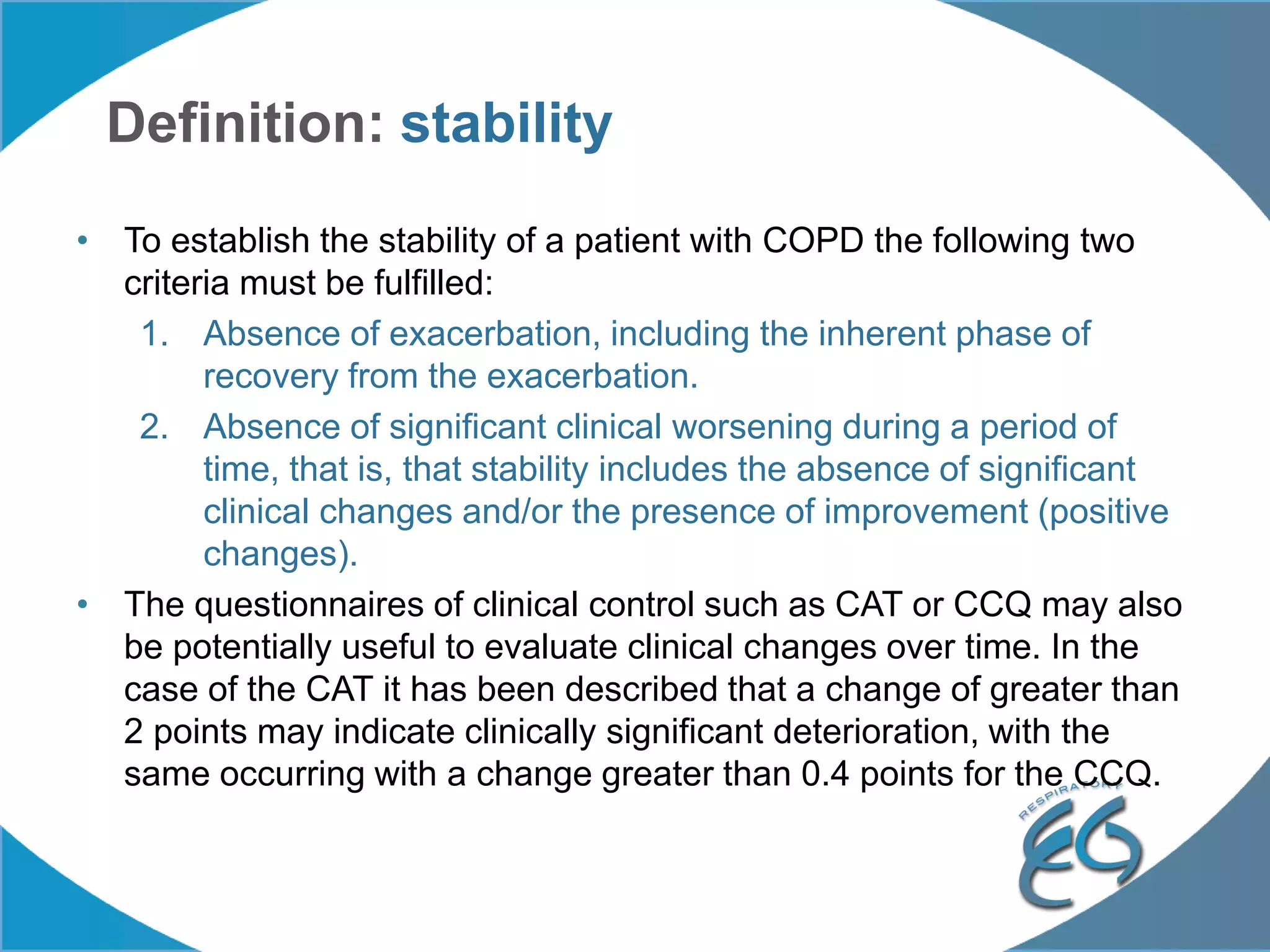
1. The REG COPD Control Working Group met on May 17th in Denver, Colorado to discuss plans to validate the concept of control in COPD through several research studies.
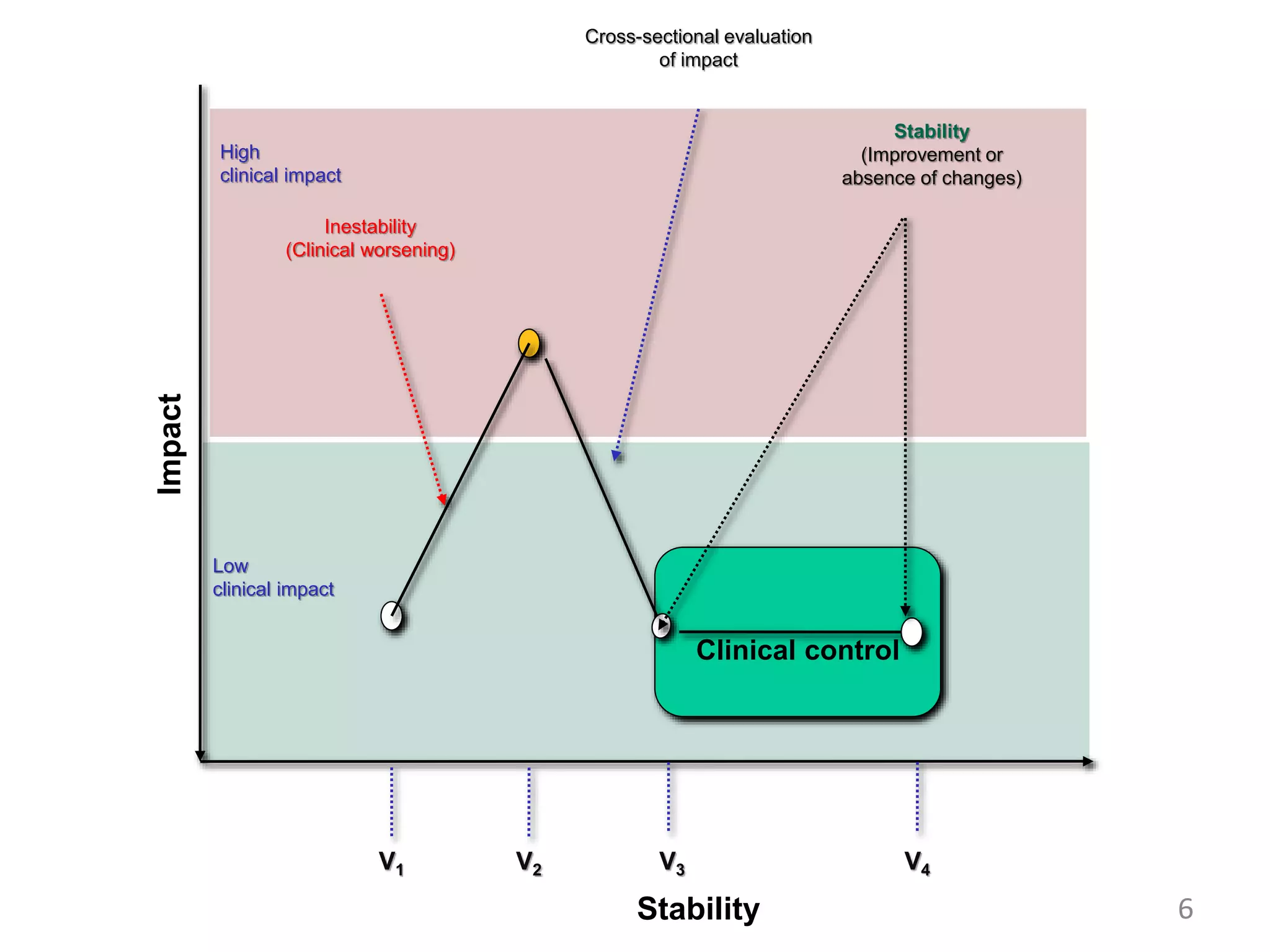
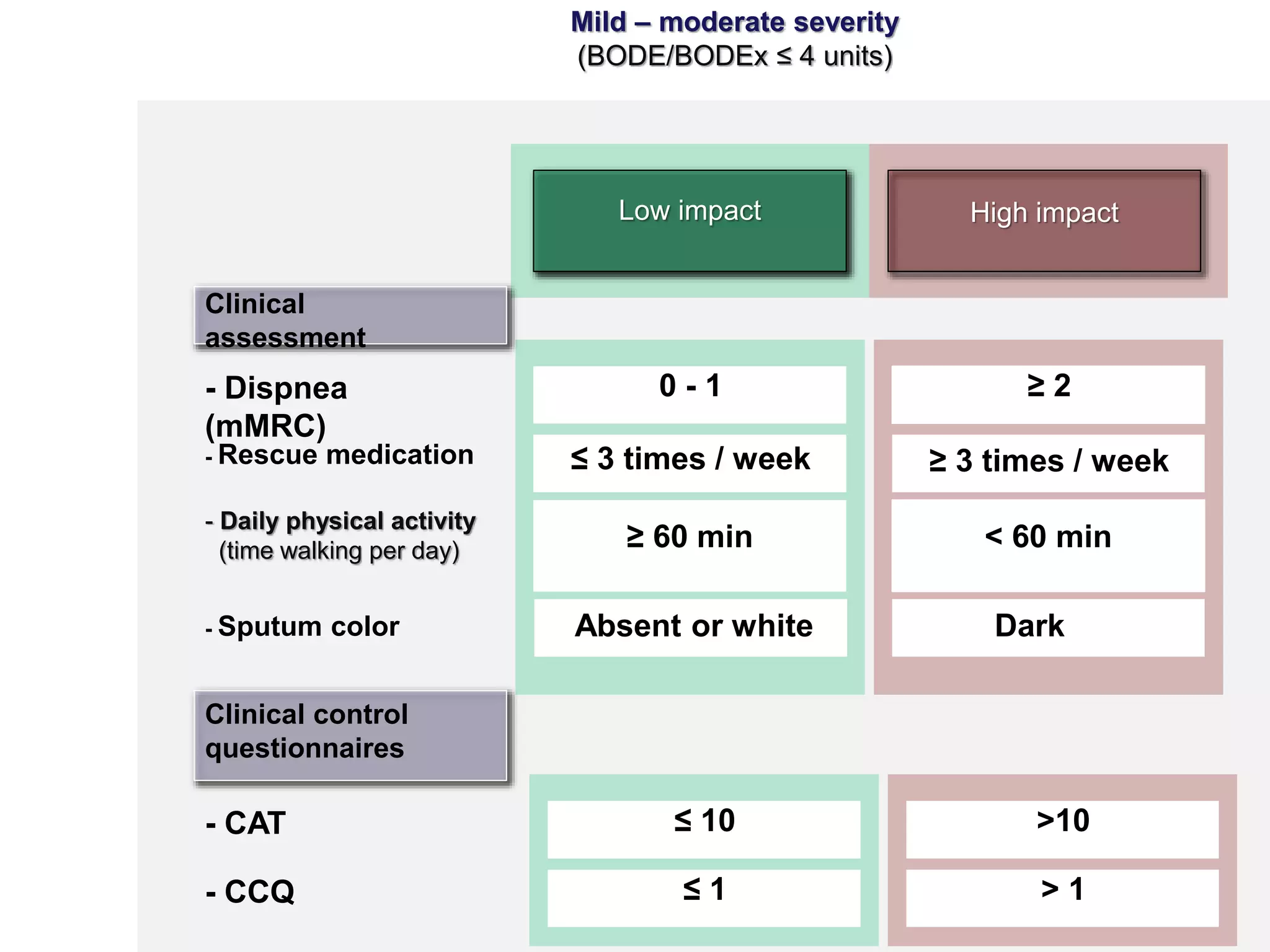
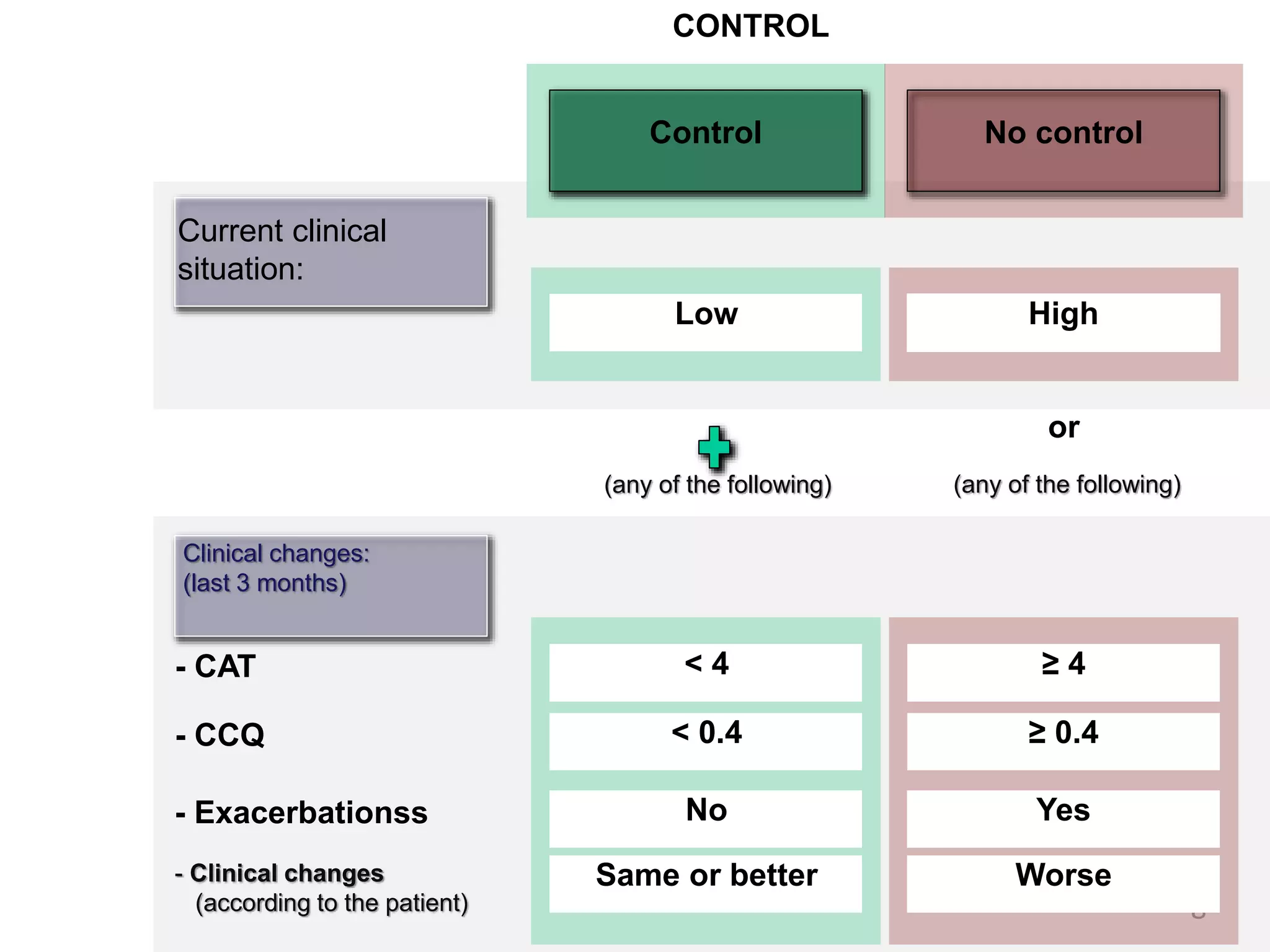
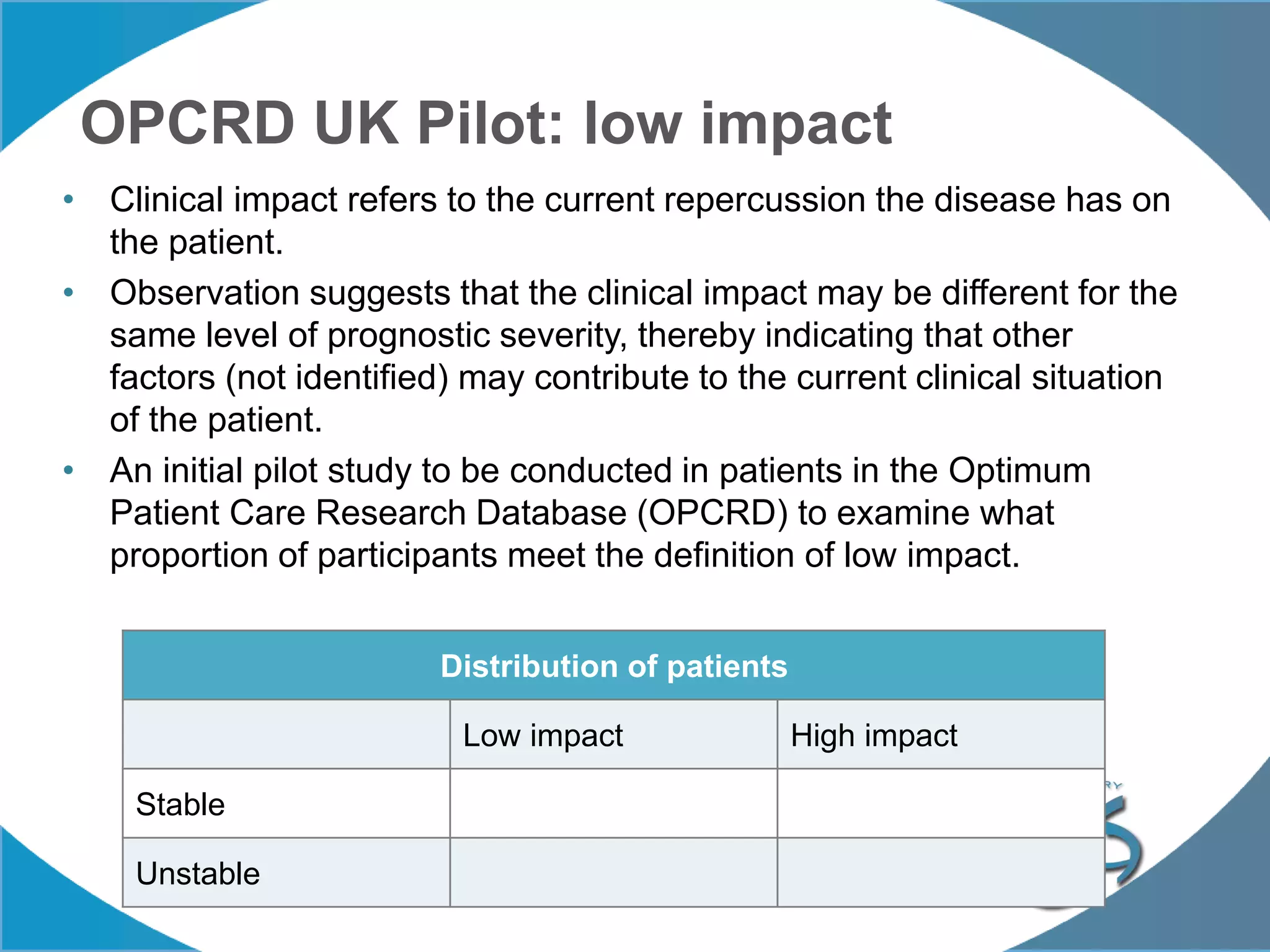
2. These included a non-interventional database study using the UK OPCRD, two Spanish pilot studies on changes in control versus severity and symptoms, and an international prospective study to validate the concept of control.
3. The group discussed objectives, timelines, and plans for implementation of these validation studies, as well as identifying new areas of research and disseminating results. The goal was to establish control as a valid concept that could help guide treatment decisions and motivate patients.