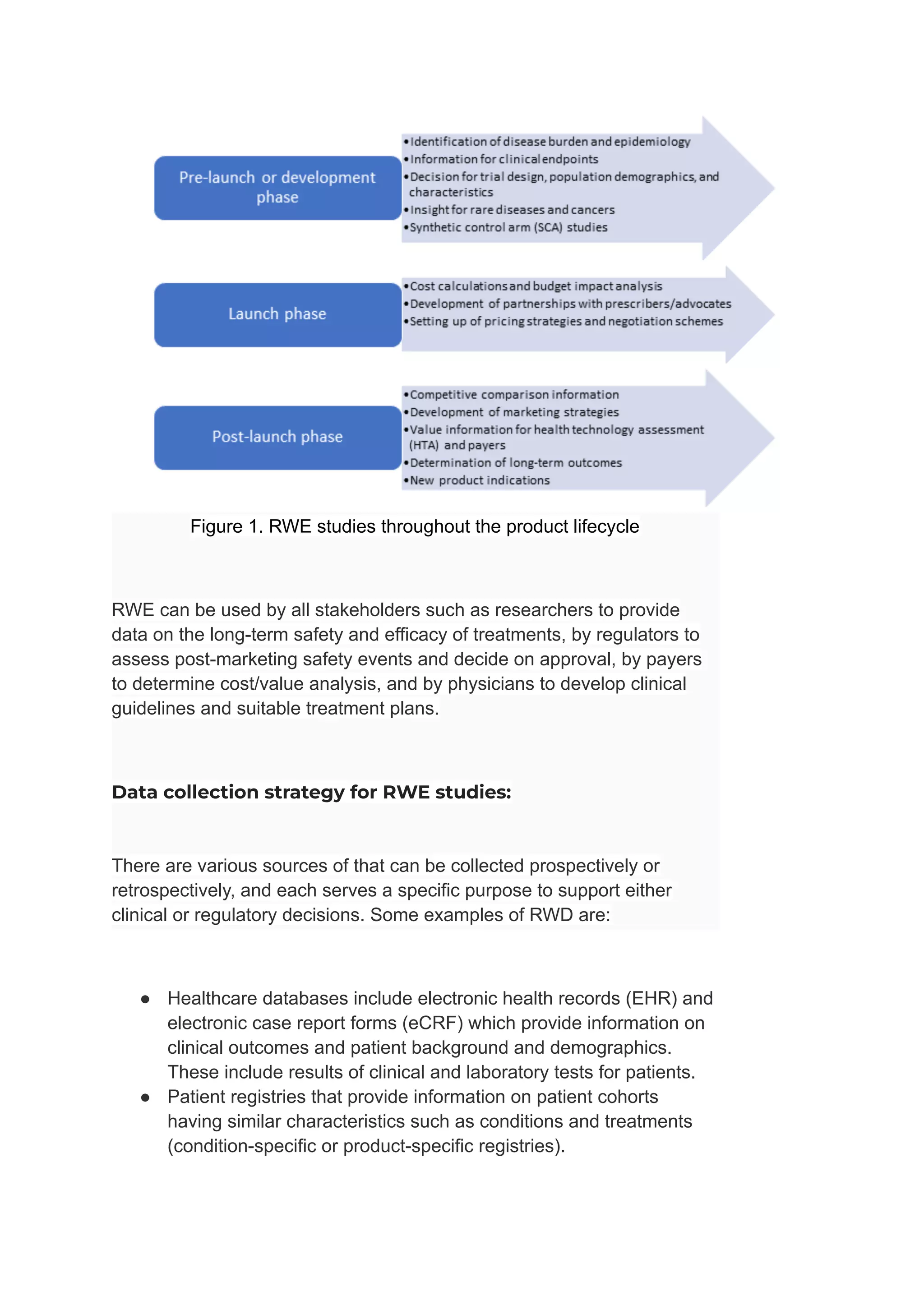
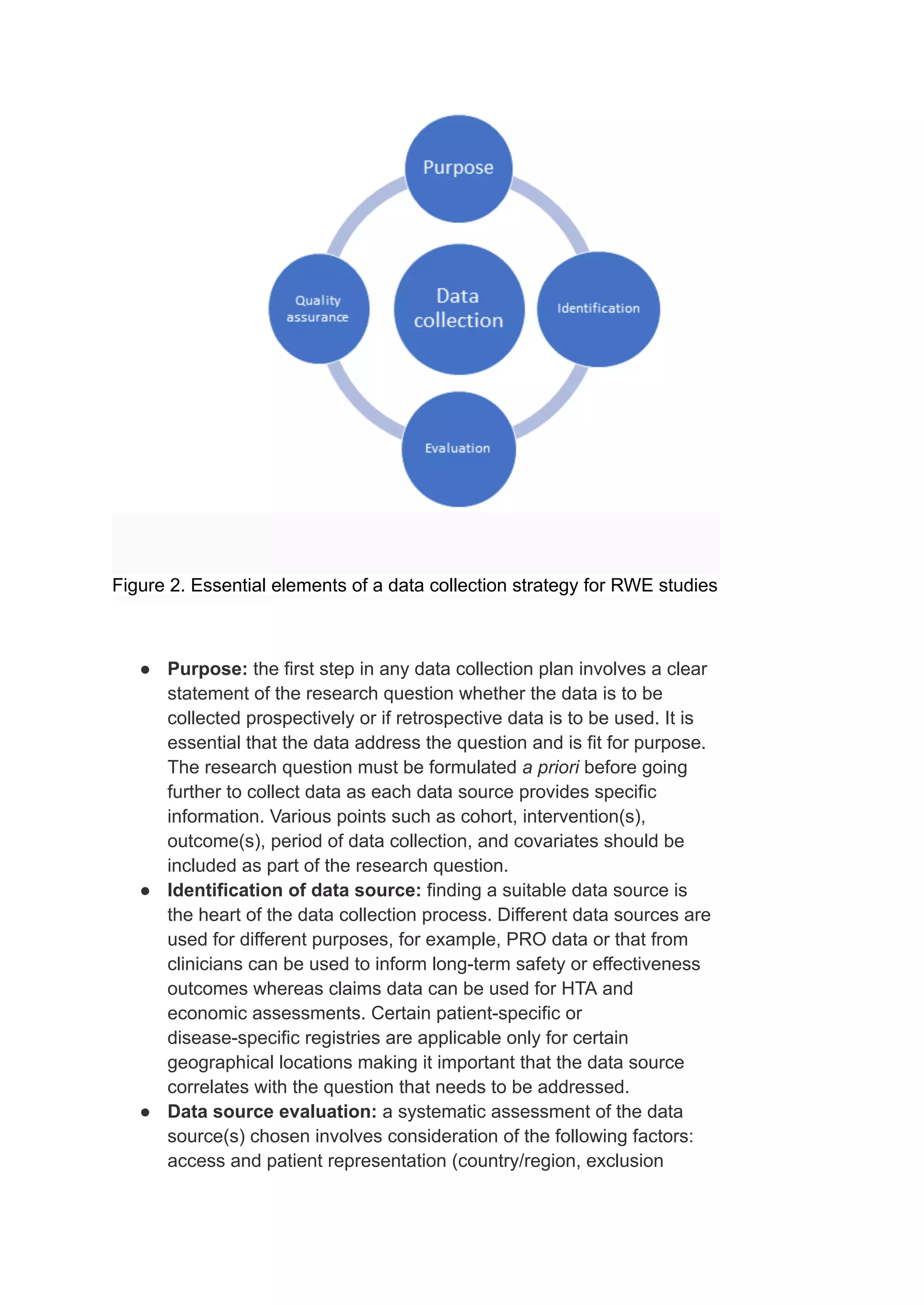
Real-world evidence (RWE) studies are increasingly utilized to complement randomized controlled trials (RCTs) by providing insights into the safety and efficacy of treatments in broader patient populations. RWE is derived from various sources such as electronic health records and patient registries, and informs decisions throughout the product lifecycle, including regulatory approvals and cost analyses. However, the reliability of RWE depends on a rigorous data collection strategy that addresses issues of data quality, representation, and appropriateness for specific research questions.