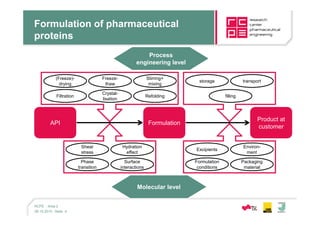
This document summarizes the work of Area II - Products and Structures at the K1 Competence Center. Area II focuses on experimental and computational research to understand product quality, behavior, and structuring methods like nanoparticle formation. The area is divided into research on large molecule drugs and small molecule drugs. The goals for both include developing stable formulations, drug delivery systems, and understanding interactions. The document provides overviews of the areas of expertise, services offered, and fields of research, which include pharmaceutical development, protein engineering, formulation, stability testing, and computational modeling.