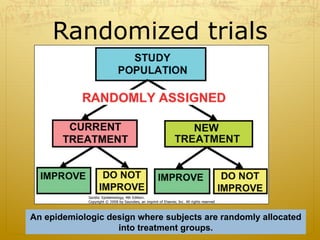
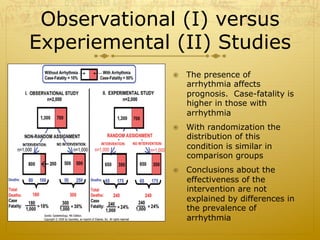
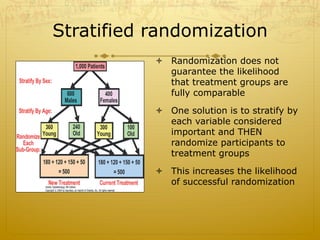
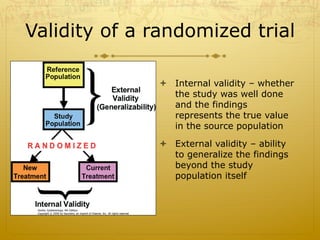
This document discusses randomized trials, including their uses in evaluating new treatments and prevention methods. It defines key concepts like randomization, stratified randomization, and masking. Randomized trials randomly assign subjects to treatment groups to reduce bias and make groups comparable. Issues like noncompliance, sample size calculations, and ethical concerns regarding informed consent are also addressed.