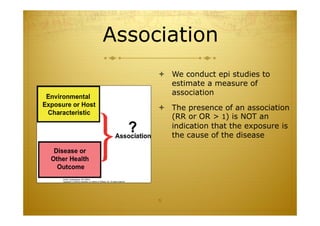
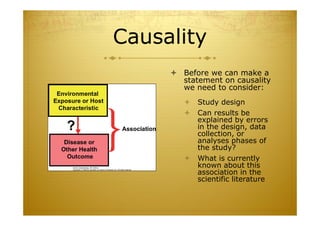
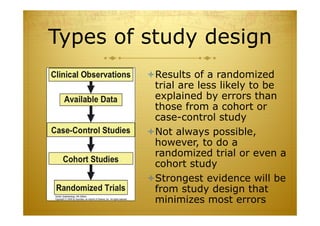
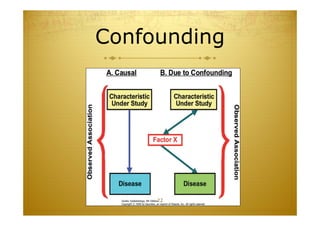
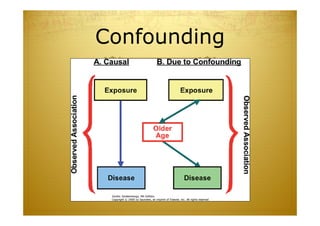
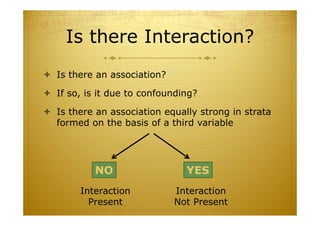
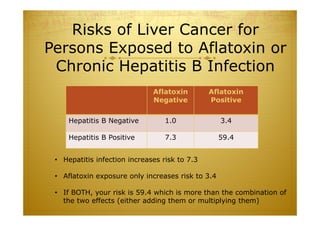
This document discusses causal inference in epidemiology. It covers objectives of understanding bias, confounding, and interaction; guidelines for assessing causality; and the goal of epidemiologic studies to test associations between exposures and outcomes. Key points are that association does not necessarily mean causation, and biases, confounding, and interactions must be considered before making causal claims. Guidelines for assessing causality include strength of association, consistency, specificity, temporality, and biological plausibility.