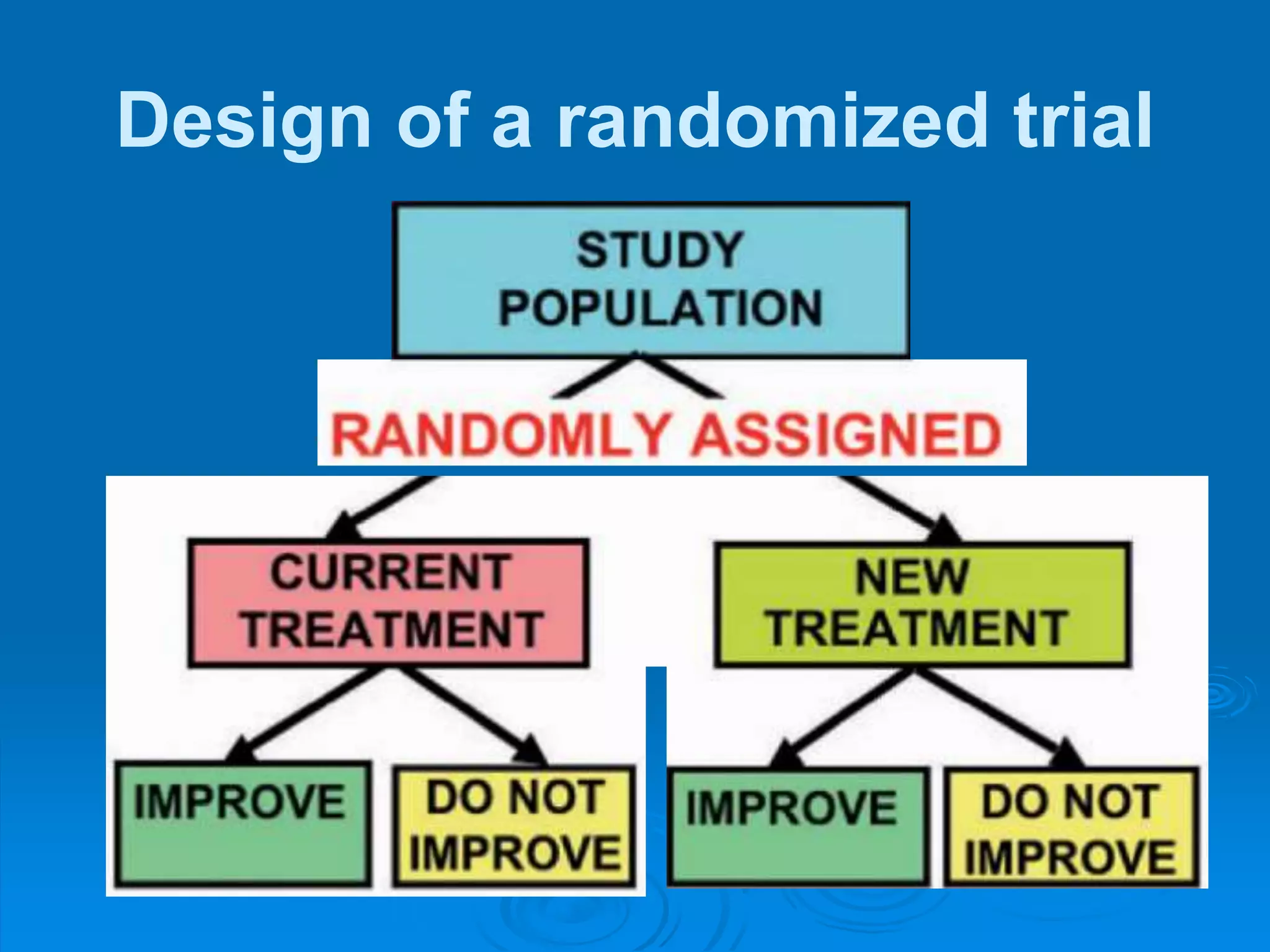
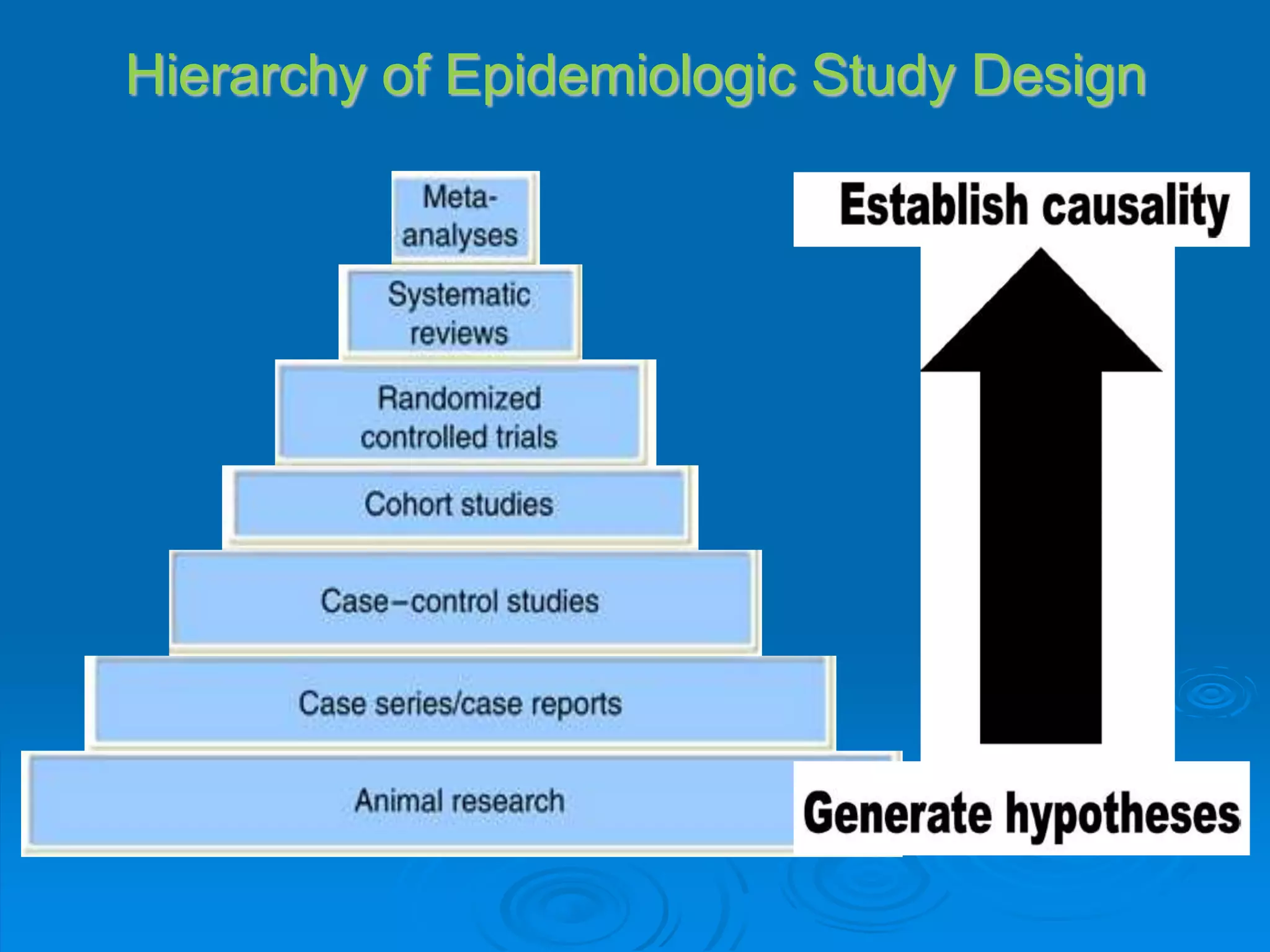
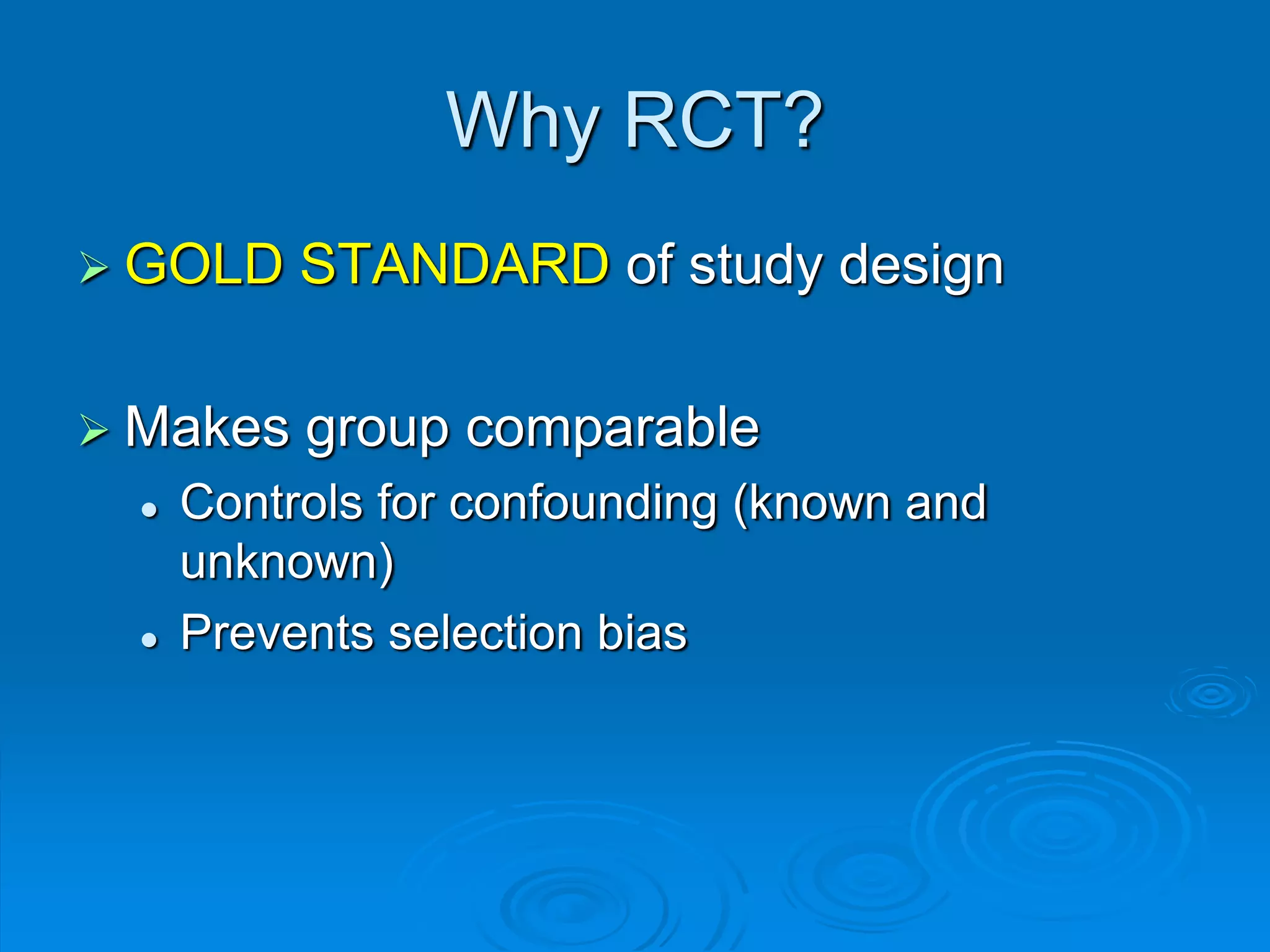
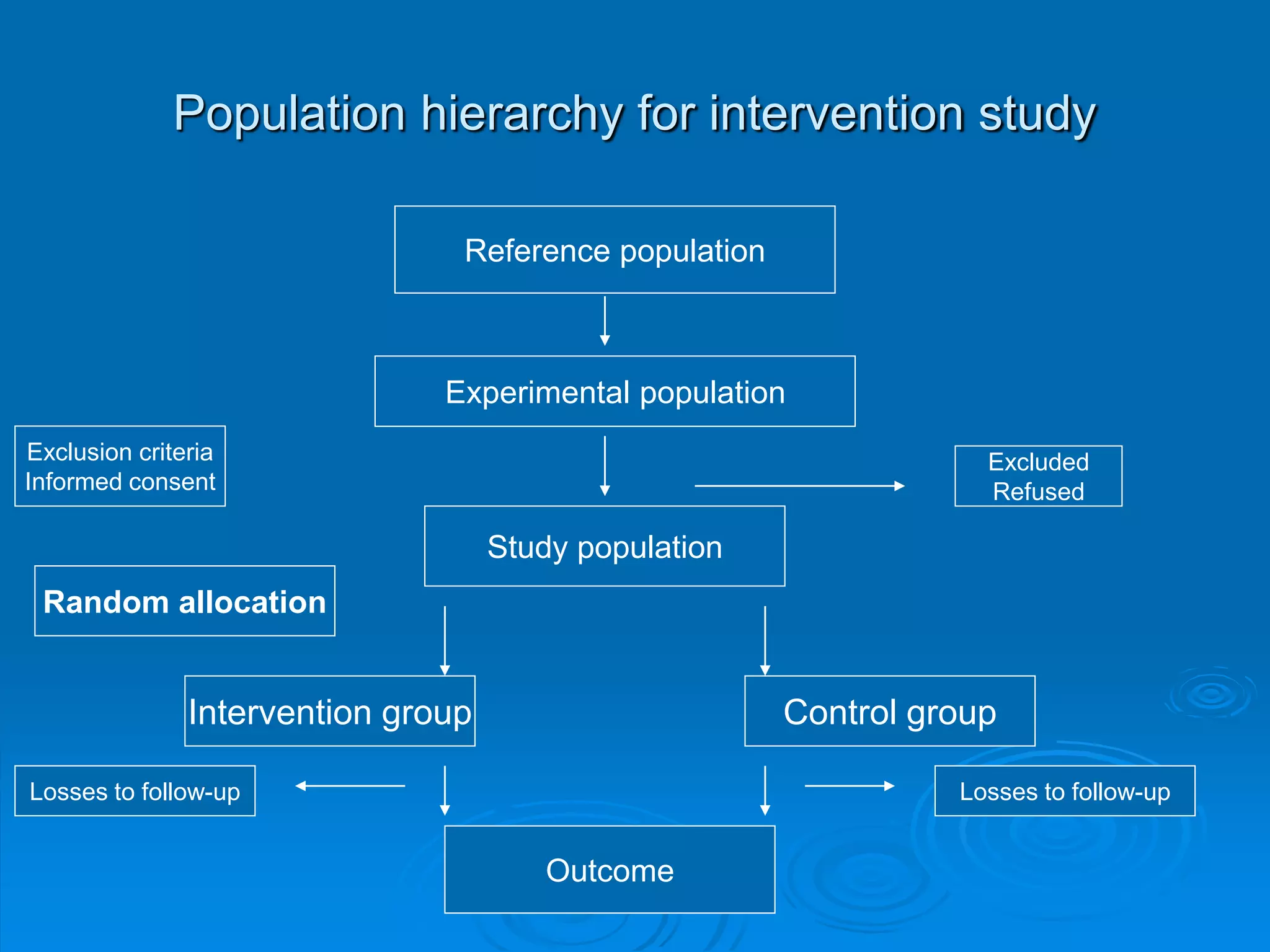
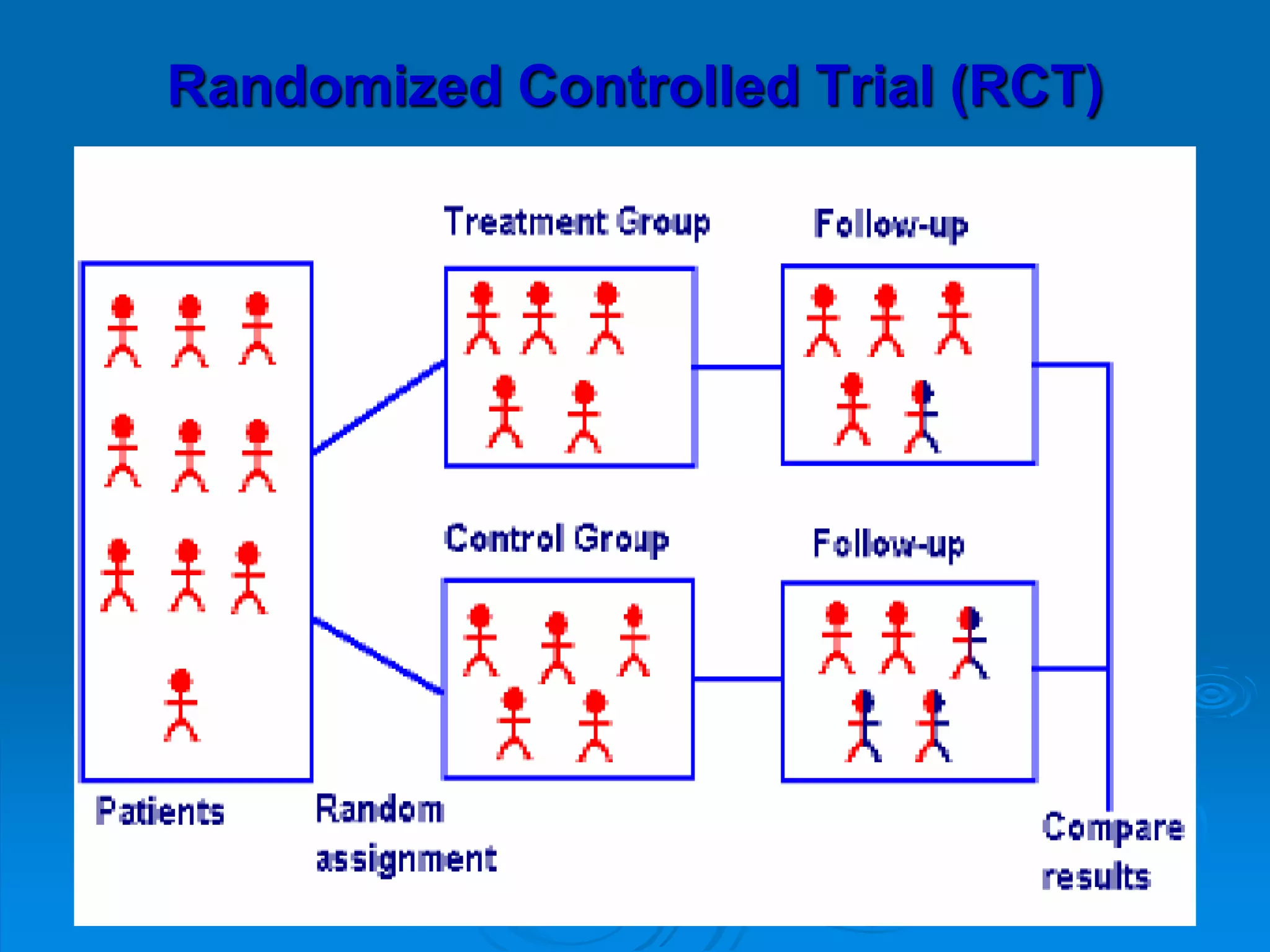
This document discusses different types of epidemiological studies, with a focus on experimental studies and randomized controlled trials (RCTs). It describes the key features of RCTs, including that they: (1) involve randomly allocating subjects into study and control groups to receive or not receive an intervention, (2) aim to control for confounding factors through randomization, and (3) are considered the gold standard for evaluating interventions due to their ability to minimize bias. The document outlines the basic steps in conducting an RCT, from developing a protocol to randomization, intervention, follow-up, assessment and analysis. It also discusses types of RCTs and their importance in evaluating treatments, prevention, risk factors and more.