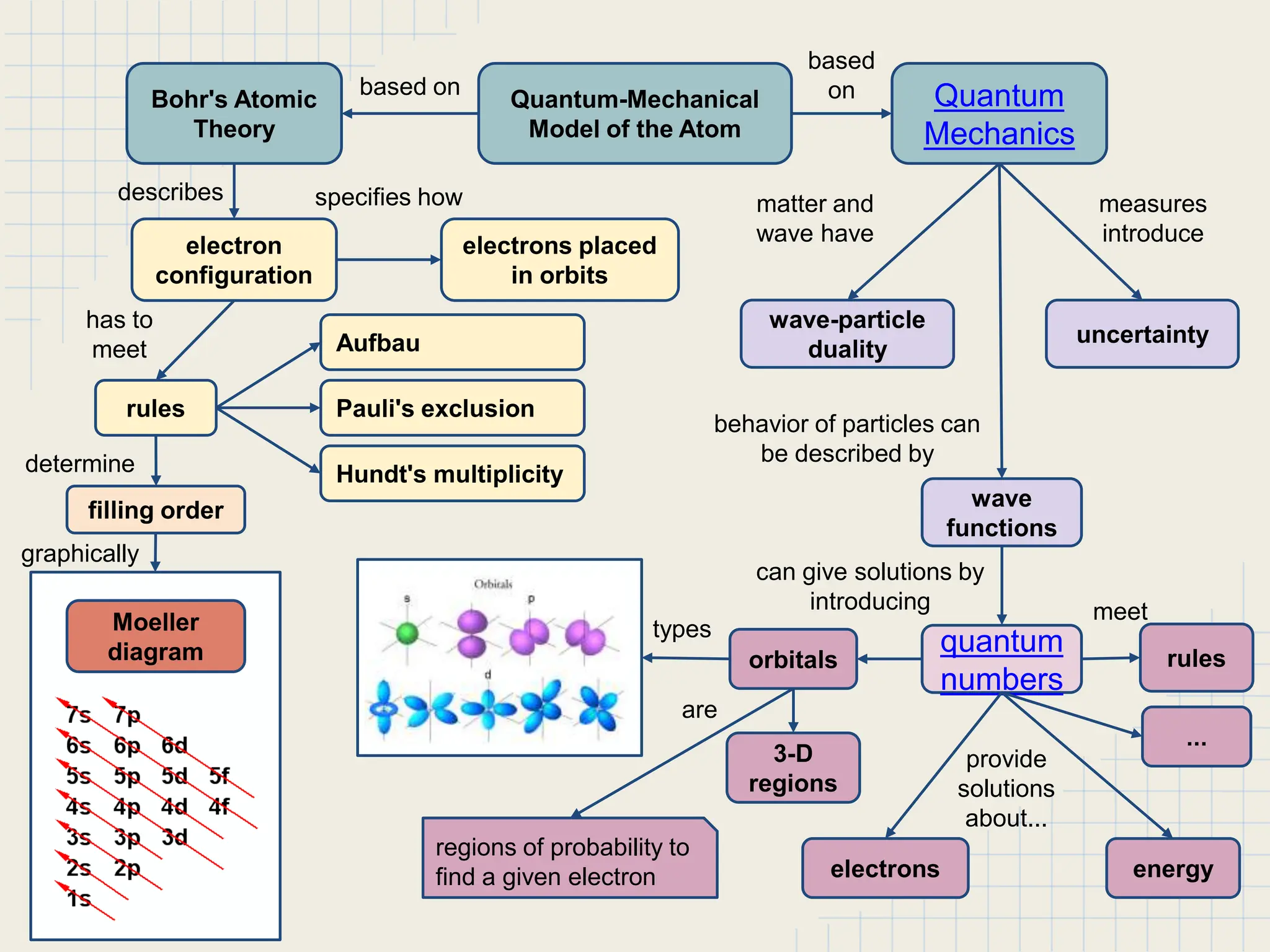
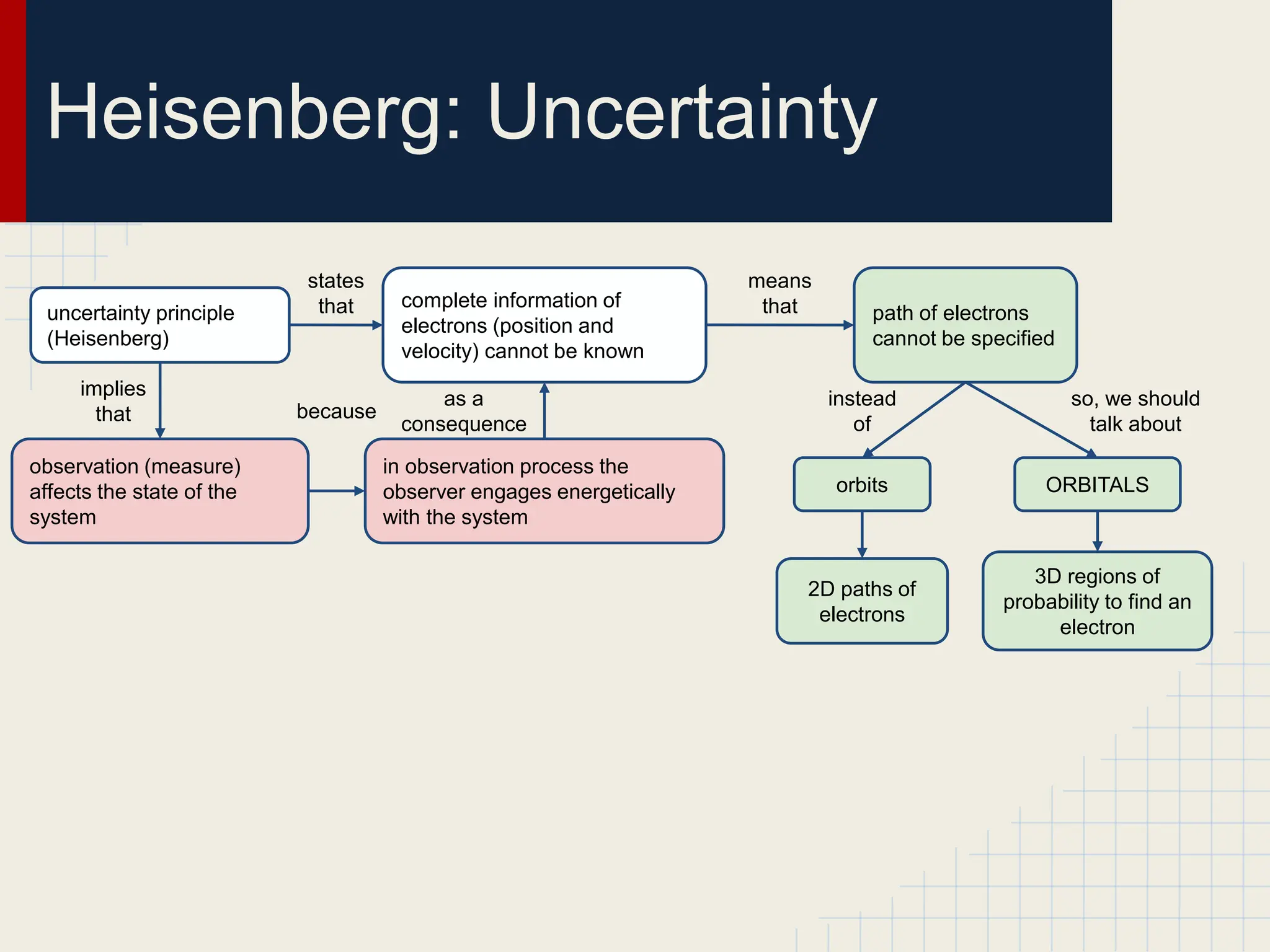
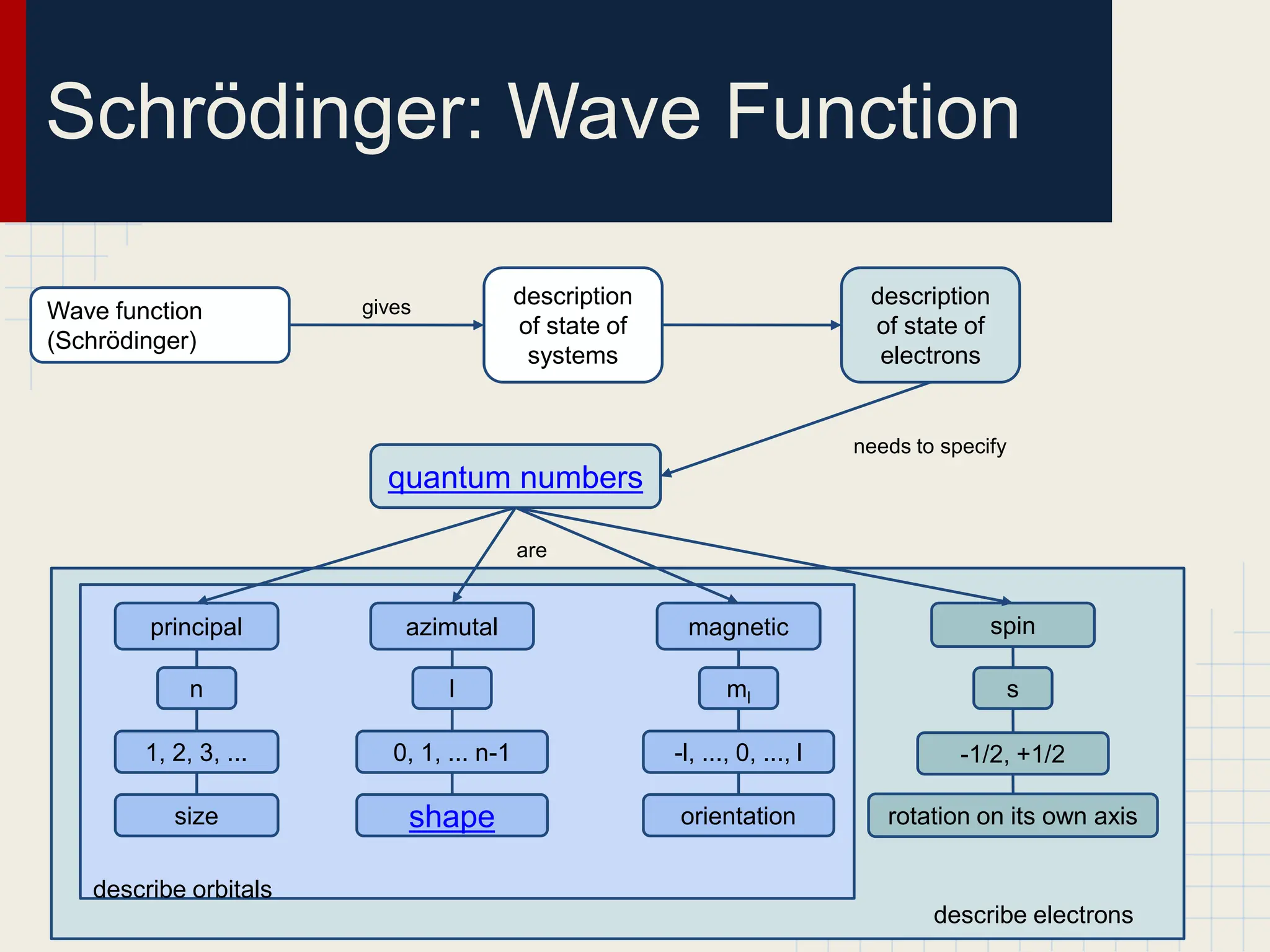
This document provides an overview of the quantum-mechanical model of the atom. It introduces key concepts like Bohr's atomic theory, quantum mechanics, electron configuration, and the uncertainty principle. The document explains that quantum mechanics describes the behavior of particles using quantum numbers and wave functions rather than definite orbits. It also discusses Schrodinger's wave function and how it provides a description of an electron's state using quantum numbers like principal, azimuthal, magnetic, and spin quantum numbers.