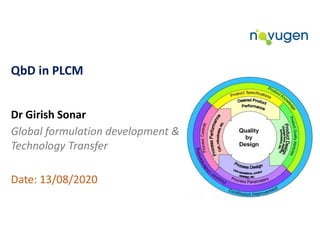
Quality by Design (Qbd) in Product Life Cycle Management (PLCM)
- 1. QbD in PLCM Dr Girish Sonar Global formulation development & Technology Transfer Date: 13/08/2020
- 2. Confidential2 Disclaimer Any views or opinions expressed herein are solely those of the author and do not necessarily represent those of any company. This presentation is solely for educational purposes and provides information for beginner scientist to share experience. For a complete requirements details, please consult to regulatory expert and/or the relevant regulatory agency.
- 3. Confidential3 Discussion point 1. Introduction to QbD 2. QbD elements – Flow diagram 3. Misconception about QbD 4. Product Development Strategy 5. Initial batches 6. QbD Stage I, II and III 7. Conclusion
- 4. Confidential4 Discussion point 1. Introduction to QbD 2. QbD elements – Flow diagram 3. Misconception about QbD 4. Product Development Strategy 5. Initial batches 6. QbD Stage I, II and III 7. Conclusion
- 5. Confidential5 Product Lifecycle Management (PLCM) Pharmaceutical Development Technology Transfer Commercial Manufacturing Product Discontinuation Product Launch Product Discontinue Reference: ICH Q10 Pharmaceutical Quality System
- 7. Confidential7 Introduction to QbD Systematic and proactive approach to pharmaceutical development Begins with predefined objectives (Define QTPP, Identify CQAs) Emphasizes product and process understanding and process control (Identify CMA & CPP and establish link between CMA/CPP to CQA) Based on sound science and quality risk management (Science- driven development - scientific literature, prior knowledge, DOEs etc. and Risk-based development - ICH Q9) Reference: ICH Q8 Pharmaceutical Development
- 8. Confidential8 Benefits of QbD (1) Ensure higher level of assurance of product quality for patient Improved product and process design & understanding Monitoring, tracking, trending of product & process. More efficient regulatory oversight Rapid introduction of state-of-the art science and technology Encouraged continuous manufacturing process improvements
- 9. Confidential9 Benefits of QbD (2) Real-time quality control and reduced end-product release testing Fewer lost batches Fewer manufacturing deviations, saving costly investigative hours Reduced out-of-specification results, reducing rework Reduce post approval changes/Variations
- 10. Confidential10 Discussion point 1. Introduction to QbD 2. QbD elements – Flow diagram 3. Misconception about QbD 4. Product Development Strategy 5. Initial batches 6. QbD Stage I, II and III 7. Conclusion
- 11. Confidential11 QbD elements – Flow diagram QTPP CQA Initial Risk Assessment Updated Risk Assessment Risk Mitigation Design Space Quality Risk Management Control Stage Drug Substance Formulation Variables Process Packing
- 12. Confidential12 QbD elements – Flow diagram QTPP CQA Initial Risk Assessment Updated Risk Assessment Risk Mitigation Design Space Quality Risk Management Control Stage Drug Substance Formulation Variables Process Packing
- 13. Confidential13 Quality Target Product Profile (QTPP) Generic MUST be ‘‘essentially similar’’ to the - 1. Dosage form 2. Dose 3. Strength 4. Route of administration 5. Safety 6. Efficacy 7. Intended use 8. Indications
- 14. Confidential14 QbD elements – Flow diagram QTPP CQA Initial Risk Assessment Updated Risk Assessment Risk Mitigation Design Space Quality Risk Management Control Stage Drug Substance Formulation Variables Process Packing
- 15. Confidential15 Critical Quality Attributes (CQAs)
- 16. Confidential16 Critical Quality Attributes (CQAs)
- 17. Confidential17 QbD elements – Flow diagram QTPP CQA Initial Risk Assessment Updated Risk Assessment Risk Mitigation Design Space Quality Risk Management Control Stage Drug Substance Formulation Variables Process Packing
- 18. Confidential18 Initial Risk Assessment o Initial risk assessment of Excipients o Initial risk assessment of Packaging material
- 19. Confidential19 QbD elements – Flow diagram QTPP CQA Initial Risk Assessment Updated Risk Assessment Risk Mitigation Design Space Quality Risk Management Control Stage Drug Substance Formulation Variables Process Packing
- 20. Confidential20 Updated Risk Assessment o Updated Risk Assessment of Drug Substance o Updated risk assessment of Excipients o Updated risk assessment of Packaging material
- 21. Confidential21 QbD elements – Flow diagram QTPP CQA Initial Risk Assessment Updated Risk Assessment Risk Mitigation Design Space Quality Risk Management Control Stage Drug Substance Formulation Variables Process Packing
- 22. Confidential22 Design Space Variables vs response
- 23. Confidential23 QbD elements – Flow diagram QTPP CQA Initial Risk Assessment Updated Risk Assessment Risk Mitigation Design Space Quality Risk Management Control Stage Drug Substance Formulation Variables Process Packing
- 24. Confidential24 Quality Risk Management Process
- 25. Confidential25 QbD elements – Flow diagram QTPP CQA Initial Risk Assessment Updated Risk Assessment Risk Mitigation Design Space Quality Risk Management Control Stage Drug Substance Formulation Variables Process Packing
- 27. Confidential27 Discussion point 1. Introduction to QbD 2. QbD elements – Flow diagram 3. Misconception about QbD 4. Product Development Strategy 5. Initial batches 6. QbD Stage I, II and III 7. Conclusion
- 28. Confidential28 Misconception about QbD (1) QbD Systemic development with predetermine objective for quality product QbD contains DoE No software mandatory to establish QbD DoE Statistical technique used in interpreting sets of experiments aimed at making sound decisions DoE may be part of QbD DoE is implemented using statistical software program Conclusion : QbD and DoE are not interchangeable terminologies.
- 29. Confidential29 Misconception about QbD (2) QTPP Desired target for developmental work Components of QTPP may or may not be in specification Not in spec – Dosage form, strength In spec – Assay, impurities Does not include acceptance criteria Specifications Includes all CQAs Specification is a list of - tests, - references to analytical procedures - acceptance criteria Establishes the set of criteria to which DP should conform to be considered acceptable for its intended use Conclusion : Defining a QTPP does not mean setting all acceptance criteria or the product specifications before development work begin.
- 30. Confidential30 Misconception about QbD (3) QbD based PDR document mandatory for regulatory submission and to make it for the sake to fulfill the submission criteria X QbD is the USFDA requirement only X DoE is mandatory for QbD as mentioned in published IR/MR product example X
- 31. Confidential31 Discussion point 1. Introduction to QbD 2. QbD elements – Flow diagram 3. Misconception about QbD 4. Product Development Strategy 5. Initial batches 6. QbD Stage I, II and III 7. Conclusion
- 33. Confidential33 Discussion point 1. Introduction to QbD 2. QbD elements – Flow diagram 3. Misconception about QbD 4. Product Development Strategy 5. Initial batches 6. QbD Stage I, II and III 7. Conclusion
- 34. Confidential34 Initial Development (Feasibility) Batches
- 35. Confidential35 Discussion point 1. Introduction to QbD 2. QbD elements – Flow diagram 3. Misconception about QbD 4. Product Development Strategy 5. Initial batches 6. QbD Stage I, II and III 7. Conclusion
- 41. Confidential41 Explanation Development batches • Formula optimization • Process optimization (partial) Scale-up batches • Process optimization (Scale dependent process) Bio batches • No optimization Commercial batches • No optimization
- 42. Confidential42 Process Optimization Process optimization planned based on knowledge of – 1.Scale dependent equipment/Process parameter 2.Scale independent equipment/Process parameter If R&D scale and commercial scale equipment have same mechanism, same geometry and scalable based on scientific basis, then process optimization batches can be performed in R&D scale equipment. If not, then process optimization batches will be performed in commercial scale equipment.
- 43. Confidential43 Process Optimization – Study Plan (1) Equipment Scalable process parameters Recommended Remark Wurster (Bottom spray) Spray rate, atomization air pressure, air flow volume, dew point ADP area is considered to calculate the scale up factor and apply to all critical process parameter except dew point and product temp Scale independent RMG Impeller speed , Chopper speed, Binder addition time, Granulation time Tip speed, Low speed = 3.0-3.5m/Sec, High Speed = 6.0-7.0m/Sec at the R&D scale and commercial scale Scale independent FBP (Top Spray granulation) Spray rate, atomization air pressure, air flow volume, dew point Calculate the scale up factor based on vendor’s recommendation and apply for critical process parameters Scale independent Multimill Milling screen opening, mill speed and direction Screen size/impeller direction/ mill speed should be same Scale independent Co- mill screen opening, mill speed and direction Screen size/impeller direction/ mill speed should be same. Apply scale factor as per vendor’s recommendation Scale independent
- 44. Confidential44 Process Optimization – Study Plan (2) Equipment Scalable process parameters Recommended Remark Blender No of revolutions, Blender geometry Blending : 300 ± 10 revolutions, Lubrication: 50 ± 5 revolutions. Calculate the blender rpm and time based on Froude no calculation. Scale independent Roller Compaction Roller speed, roller gap, compaction force, milling parameters Scaling up factor varies from mechanism of roller compaction and follow vendor’s guideline for scale-up Scale independent most of the time Compression machine Turret speed, feeder speed, pre-compression force, main compression force, dwell time Optimize the process parameters wrt compression machine at manufacturing site Scale dependent Coating Spray rate, atomization air pressure, product temp, gun to bed distance, pan rpm Optimize the process parameters wrt coating machine at manufacturing site Scale dependent
- 46. Confidential46 QbD Stage II QbD II Stage = Updated risk assessment with justification based on development batches results
- 48. Confidential48 Quality Risk Management Risk Review RiskCommunication Risk Assessment Risk Evaluation unacceptable Risk Control Risk Analysis Risk Reduction Risk Identification Review Events Risk Acceptance Initiate Quality Risk Management Process Output / Result of the Quality Risk Management Process RiskManagementtools
- 49. Confidential49 Risk Management Tools 1. Basic risk management facilitation methods (flowcharts, check sheets etc.) 2. Failure Mode Effects Analysis (FMEA) 3. Failure Mode, Effects and Criticality Analysis (FMECA) 4. Fault Tree Analysis (FTA) 5. Hazard Analysis and Critical Control Points (HACCP) 6. Hazard Operability Analysis (HAZOP) 7. Preliminary Hazard Analysis (PHA) 8. Risk ranking and filtering 9. Supporting statistical tools
- 50. Confidential50 Basic Risk Management Facilitation Method 1. Flowcharts; 2. Check Sheets; 3. Process Mapping; Cause and Effect Diagrams (also called an Ishikawa diagram or fish bone diagram)
- 51. Confidential51 FMEA – Case Study (1) Risk Assessment Risk Reduction Source Failure mode Effect Severity Cause Occurrence Current Controls Delectability RPN Action Plan Severity Occurrence Delectability RPN Document reference Remark Granulation Dry mixing speed slow May not meet the specifications for Blend uniformity, content uniformity and Drug release 7 Mixing speed of agitator not within acceptable range (50±5RPM) 4 Instruction for standard mixing speed and time given in the BMR 4 112 Four eye principle, Documentatio n for the same in BMR with signature. 4 1 1 4 BMR Instruction s in granulation section. Risk reduced. Granulation Dry mixing Time 7 Mixing time less than or more than 5min 4 4 112 Four eye principle, Documentatio n for the same in BMR with signature. 4 1 1 4 BMR Instruction s in granulation section. Risk reduced. Granulation Time of Binder addition Not meet the physical parameters of the Blend and Blend uniformity 10 Binder added less than 1min or more than 2min 7 Instruction for standard binder addition time given in the BMR 4 280 Four eye principle, Documentatio n for the same in BMR with signature. 7 4 1 28 BMR Instruction s in granulation section. Risk reduced.
- 52. Confidential52 FMEA – Case Study (2) Risk Assessment Risk Reduction Source Failure mode Effect Severity Cause Occurrence Current Controls Delectability RPN Action Plan Severity Occurrence Delectability RPN Document reference Remark Granulation Speed of agitator Not meet the physical parameters of the Blend and Blend uniformity 7 Speed of agitator not maintain slow (50±5RPM) 4 Instruction for standard binder addition time given in the BMR. 4 112 Four eye principle, Documentatio n for the same in BMR with signature. 4 1 1 4 BMR Instruction s in granulation section. Risk reduced. Granulation Use of Chopper (not to be use) Not meet the physical parameters of the Blend and Blend uniformity 10 Chopper started. 7 Instruction for not using Chopper is given in BMR. 4 280 Four eye principle, Documentatio n for the same in BMR with signature. 4 4 1 16 BMR Instruction s in granulation section. Risk reduced. Max Average Min 280 180 112 Max Average Min 28 11 04
- 54. Confidential54 QbD Stage III QbD III Stage = Control Strategy Control strategy should be discussed with manufacturing person before finalize for the best results. All critical attributes control should be mentioned clearly in control strategy and mentioned the name of reporting documents.
- 56. Confidential56 Product Lifecycle Management (PLCM) Pharmaceutical Development Technology Transfer Commercial Manufacturing Product Discontinuation Reference: ICH Q10 Pharmaceutical Quality System Product Launch Product Discontinue
- 57. Confidential57 Discussion point 1. Introduction to QbD 2. QbD elements – Flow diagram 3. Misconception about QbD 4. Product Development Strategy 5. Initial batches 6. QbD Stage I, II and III 7. Conclusion
- 58. Confidential58 Conclusion QbD is the effective tool, should be implement from the initial stage of the product development independent of target market. Discuss QbD scheme with manufacturing team to achieve aim of QbD and keep future projection to avoid regulatory queries and post approval changes/Variation. DoE is not mandatory for QbD based submission. Try to cover maximum range of formulation and process variables during optimization study to make fastest and cost-effective post approval changes/Variation.
- 59. Confidential59
