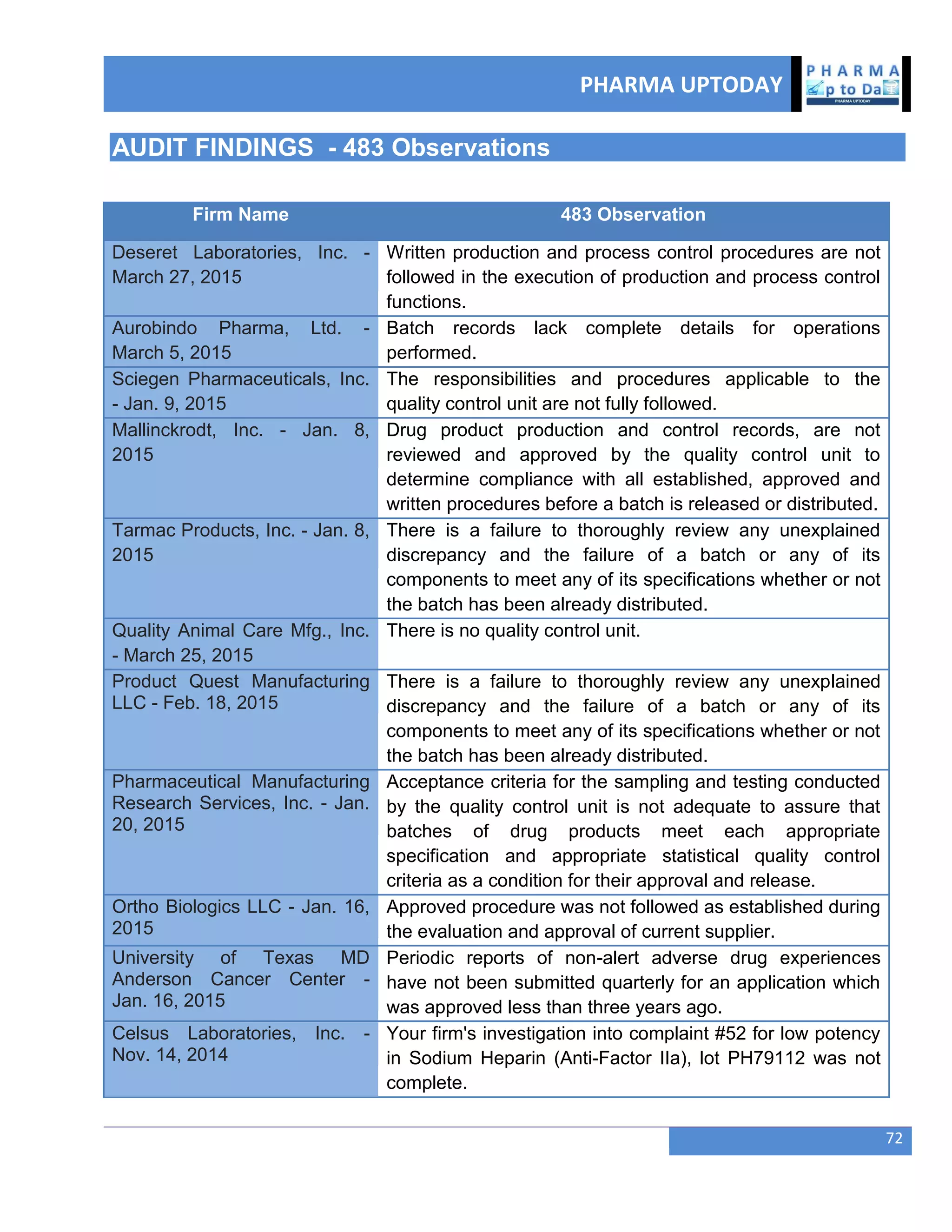
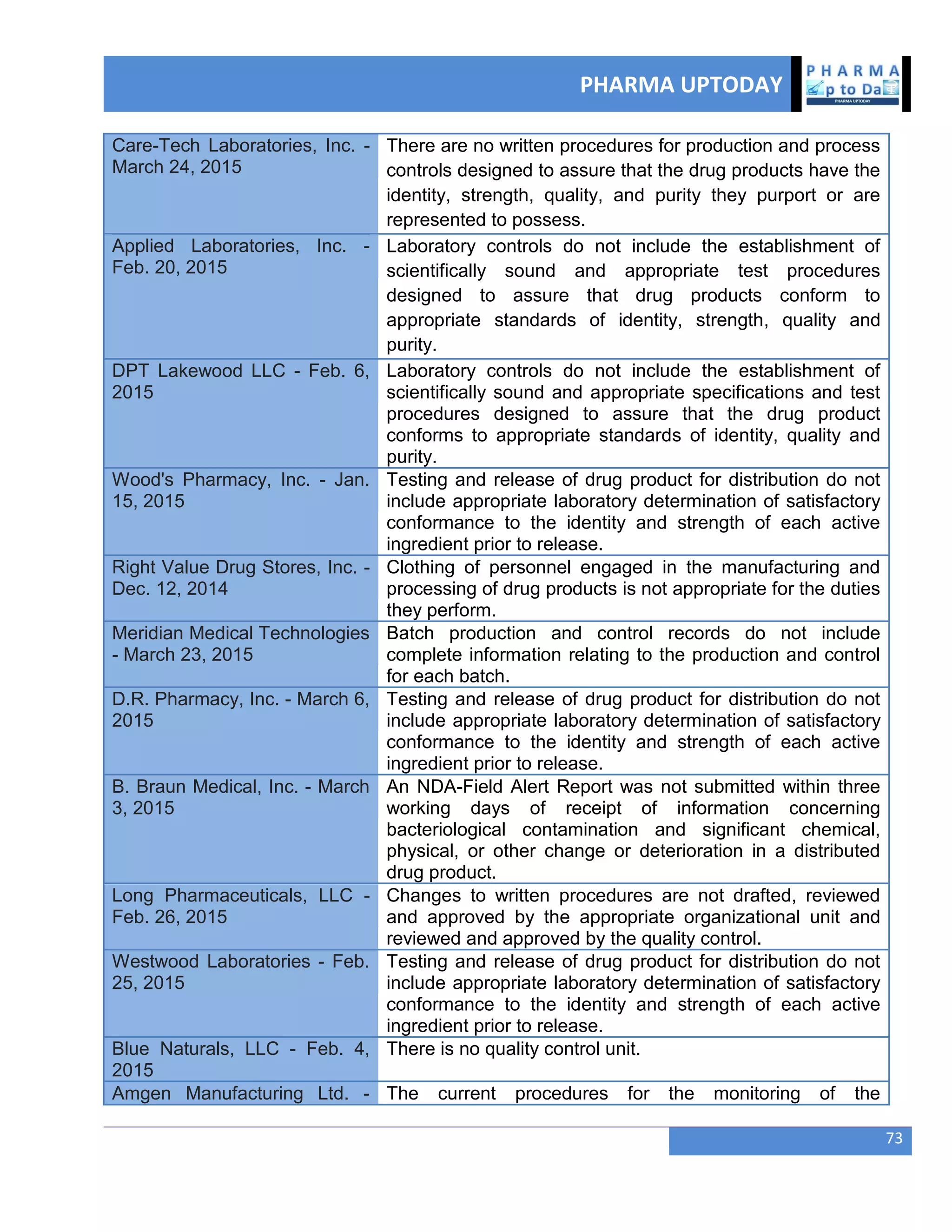
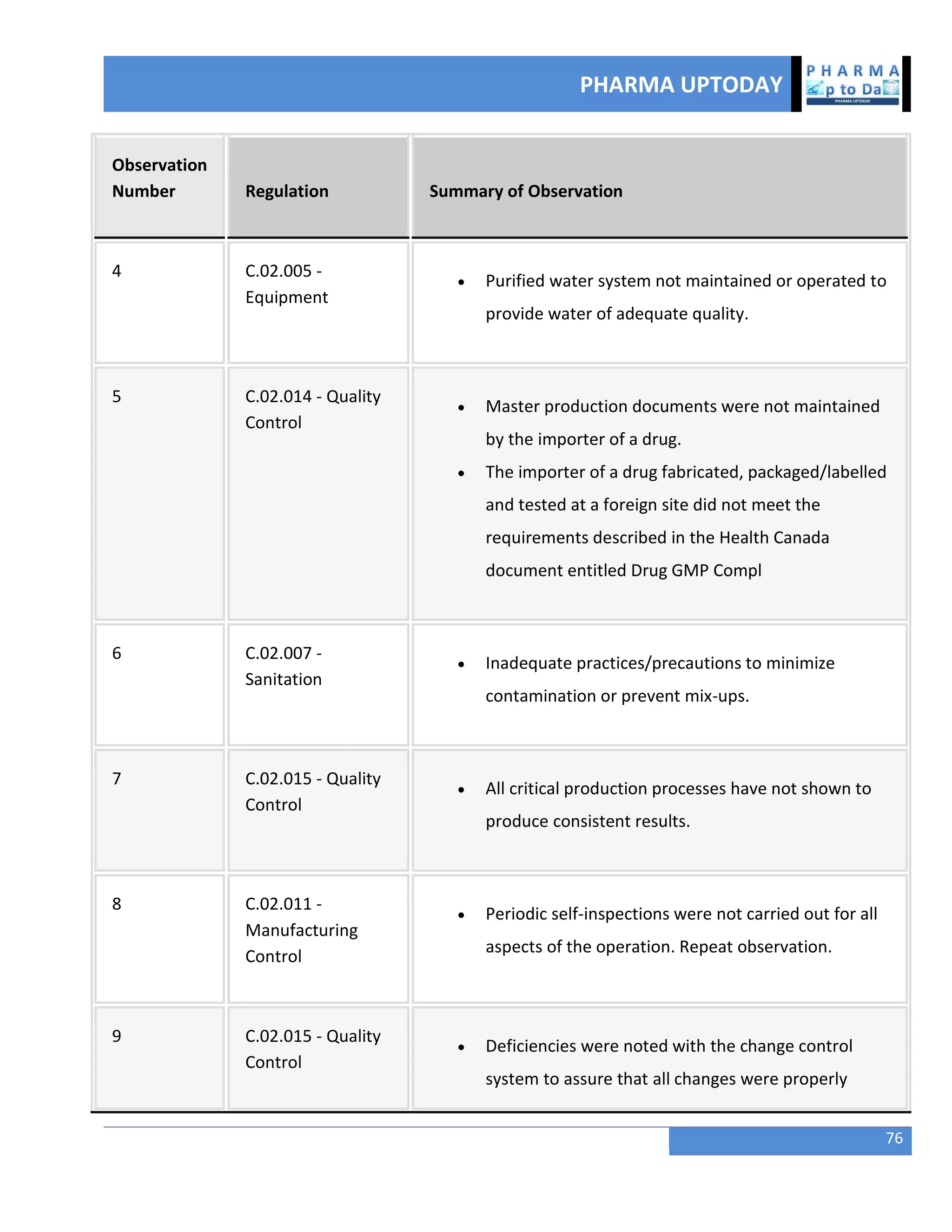
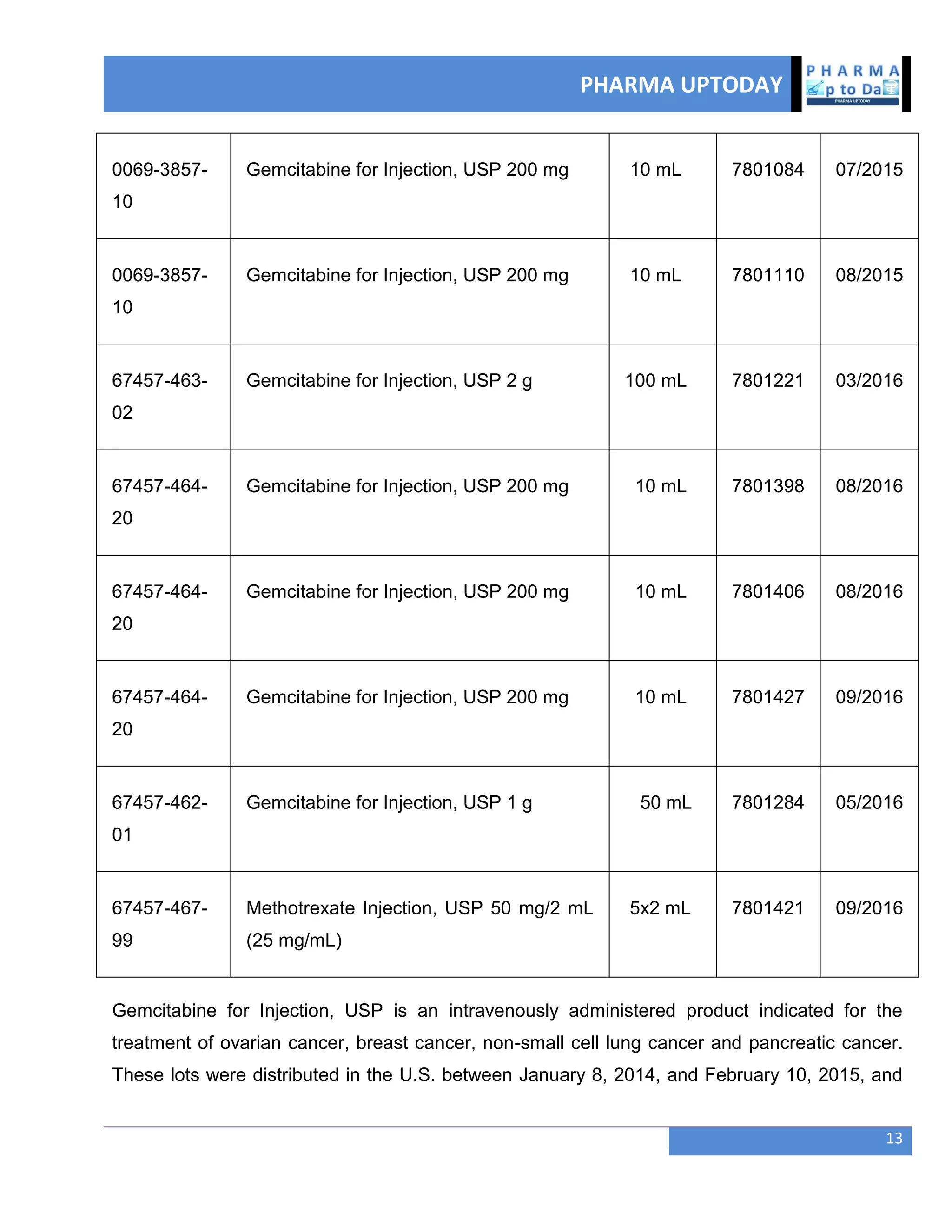
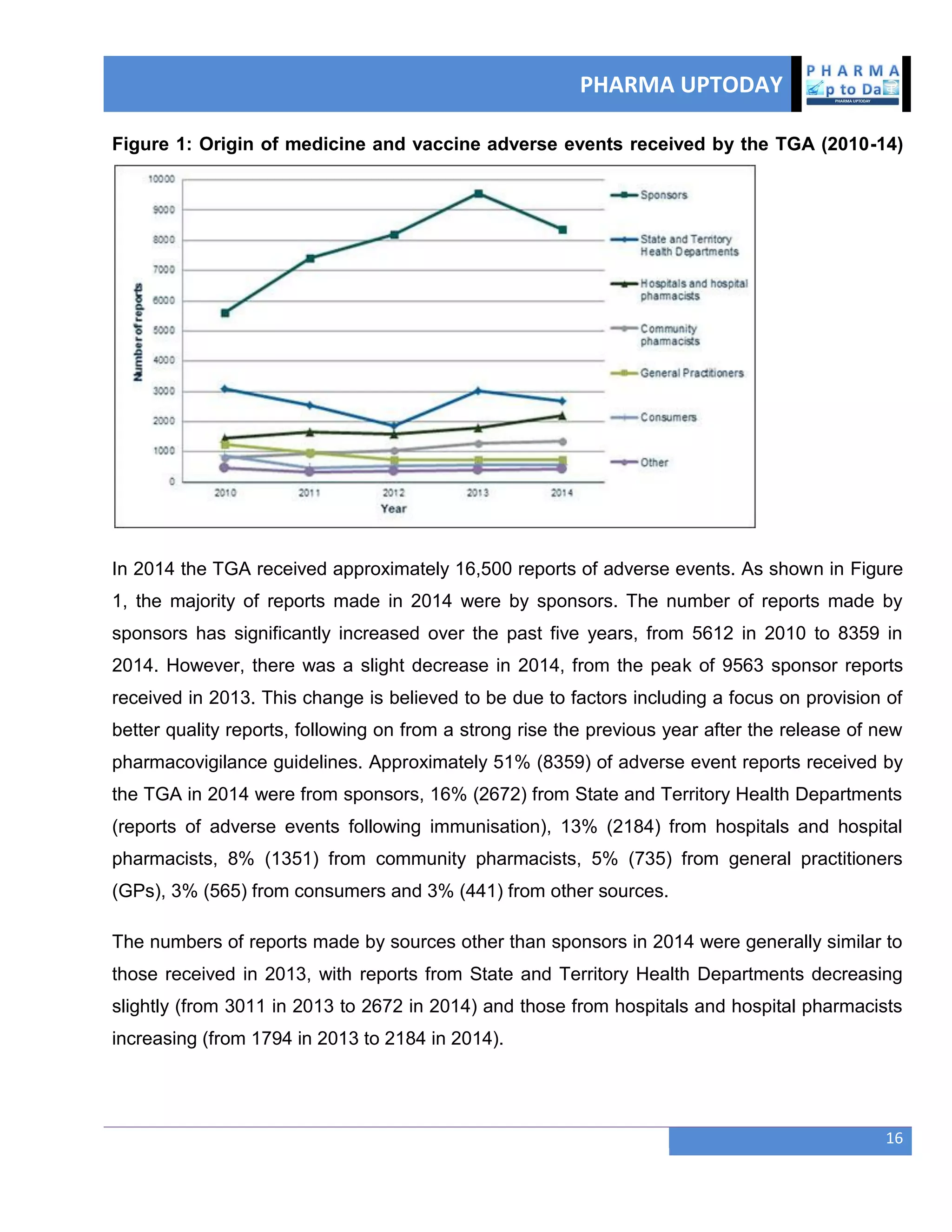
The document discusses various regulatory and quality events in the pharmaceutical sector, particularly focusing on a trip by FDA officials to China aimed at improving drug safety and regulatory collaboration. It highlights significant recalls of medications due to manufacturing deviations, as well as positive Phase III trial results for a diabetes treatment involving dapagliflozin, saxagliptin, and metformin. Additionally, Mylan is expanding a recall of select injectable products due to visible particulate matter, emphasizing the potential health risks associated with their use.







![PHARMA UPTODAY
8
For pharmaceutical manufacturers with products in this category, I strongly suggest that you
poll your wholesaler customers and find out how often they break down your multi-pack today
and sell the individual packages inside to their dispensing customers.
The DSCSA defines the term ―Package‖ this way:
―(A) IN GENERAL.—The term ‗package‘ means the smallest individual saleable unit of product
for distribution by a manufacturer or repackager that is intended by the manufacturer for
ultimate sale to the dispenser of such product.
―(B) INDIVIDUAL SALEABLE UNIT.—For purposes of this paragraph, an ‗individual saleable
unit‘ is the smallest container of product introduced into commerce by the manufacturer or
repackager that is intended by the manufacturer or repackager for individual sale to a
dispenser. [DSCSA Section 581(11)]
Yes, you could probably say that your multi-pack–just like today–is the level of packaging that
you intend for ultimate sale to the dispenser and so that‘s all you will be required to serialize.
And theoretically, you would be right. But, if today, before serialization is required, wholesalers
routinely break your multi-pack down further as part of their friendly service to their customers,
they won‘t be able to do that once they begin operating under the serialization requirements of
the DSCSA–November 27, 2019.
After that date they will no longer be able to break any package down unless the components
within are also serialized by the manufacturer or repackager.
―So what?‖ you say? If your product is routinely broken down by the wholesaler today, that is
done to supply small pharmacies with a smaller amount of your product so they are able to](https://image.slidesharecdn.com/pummvolume16issuejul2015-150723171259-lva1-app6891/75/Pharma-Uptoday-Monthly-Magazine-Volume-16-Issue-Jul-2015-8-2048.jpg)












![PHARMA UPTODAY
24
ANVISA passed Kemwell‘s manufacturing facility for oral solids in Bangalore, India following a
recent inspection. The site will now be allowed to manufacture J&J‘s TB drug Sirturo
(bedaquiline) for the Brazilian market. The facility already makes drugs for the EU, US, Canada,
South Africa, and Australia.
―The [US] FDA is tough but ANVISA is considered the toughest regulatory agency with respect
to audits,‖ Ashok Hegde, Kemwell‘s VP, Pharmaceutical Operations, told this publication.
―Every inspector has its own methodology of auditing. The FDA mostly concentrates on the
documentation part. If an audit lasts five days, four of those will be spent on documentation.
―But ANVISA is exactly the opposite – it spends most of its time focusing on the facility and live
operations. They will ask you to demonstrate things about the manufacturing procedure.
―They want to be on the ‗shop floor‘ when manufacturing is going on – to see how you do
granulation or a tabletting procedure. They want to witness everything.‖
Brazilian market
The US and Brazilian agencies also differ in their warning notice. Since the FDA opened
several offices in India in the last four years, around 12 inspectors have been based in the
country and can schedule inspections at short notice. ―They will call you on Friday and say
they‘re coming on Monday,‖ said Hegde. ANVISA typically gives four weeks‘ warning, he
said. Brazil has historically had tight rules about pharmaceutical imports. Hegde said few
foreign manufacturers are approved by ANVISA, and the region prefers home-made products
or drugs imported in bulk and then packaged for consumers in Brazil.
But the country is growing as a market for complex, high-quality drugs. Hegde pinpointed
injectables, especially biologics, and third-generation antibiotics like cephalosporin as ―the next
segments where demand is going to pick up.‖](https://image.slidesharecdn.com/pummvolume16issuejul2015-150723171259-lva1-app6891/75/Pharma-Uptoday-Monthly-Magazine-Volume-16-Issue-Jul-2015-24-2048.jpg)