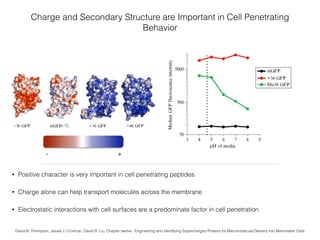
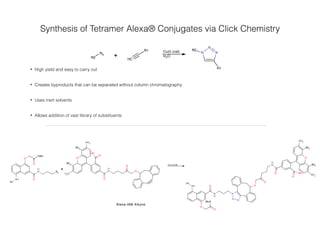
This document summarizes research on synthesizing anionic foldamers for macromolecule delivery. It discusses how charge and secondary structure influence cell penetration, and how the research group synthesized quinoline oligomers and conjugated them to Alexa fluorophores via click chemistry. The conjugates could deliver cargo like oligonucleotides, peptides, and be used to assay for successful cell penetration. The overall goal is to develop non-toxic anionic molecules that can transport therapeutic macromolecules into cells.