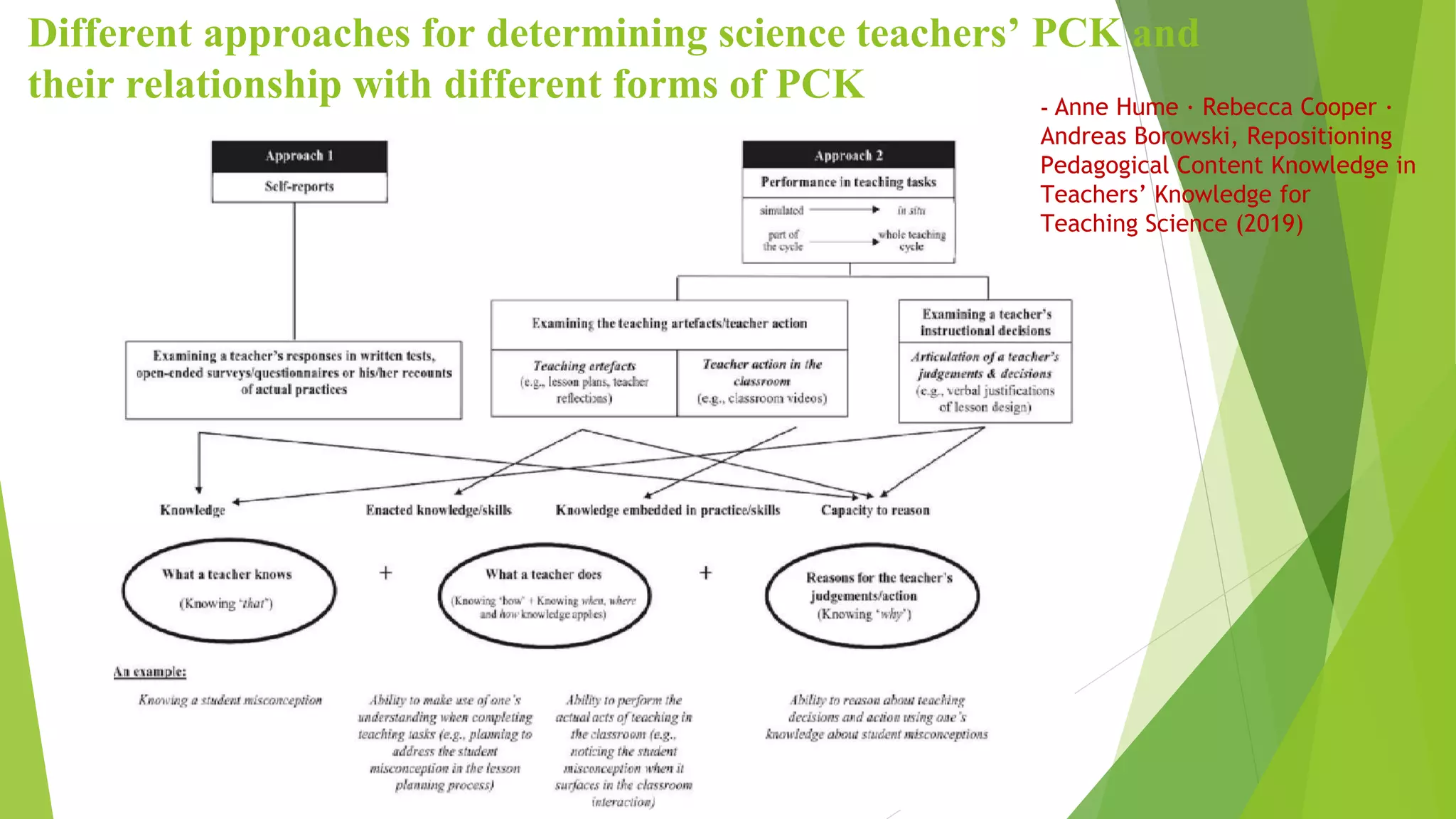
This document discusses pedagogical content knowledge (PCK) for teaching physical science concepts like physics and chemistry at the high school level. It provides an overview of the Next Generation Science Standards performance expectations and core ideas for high school physical sciences. The document also discusses definitions of PCK from various scholars and models for representing PCK, with a focus on developing PCK specifically for teaching topics like the gas laws in chemistry. It notes that teachers' representational competence in navigating between macroscopic, particulate and symbolic representations is important for developing a deep understanding of concepts like the gas laws but is not well understood based on the existing literature.