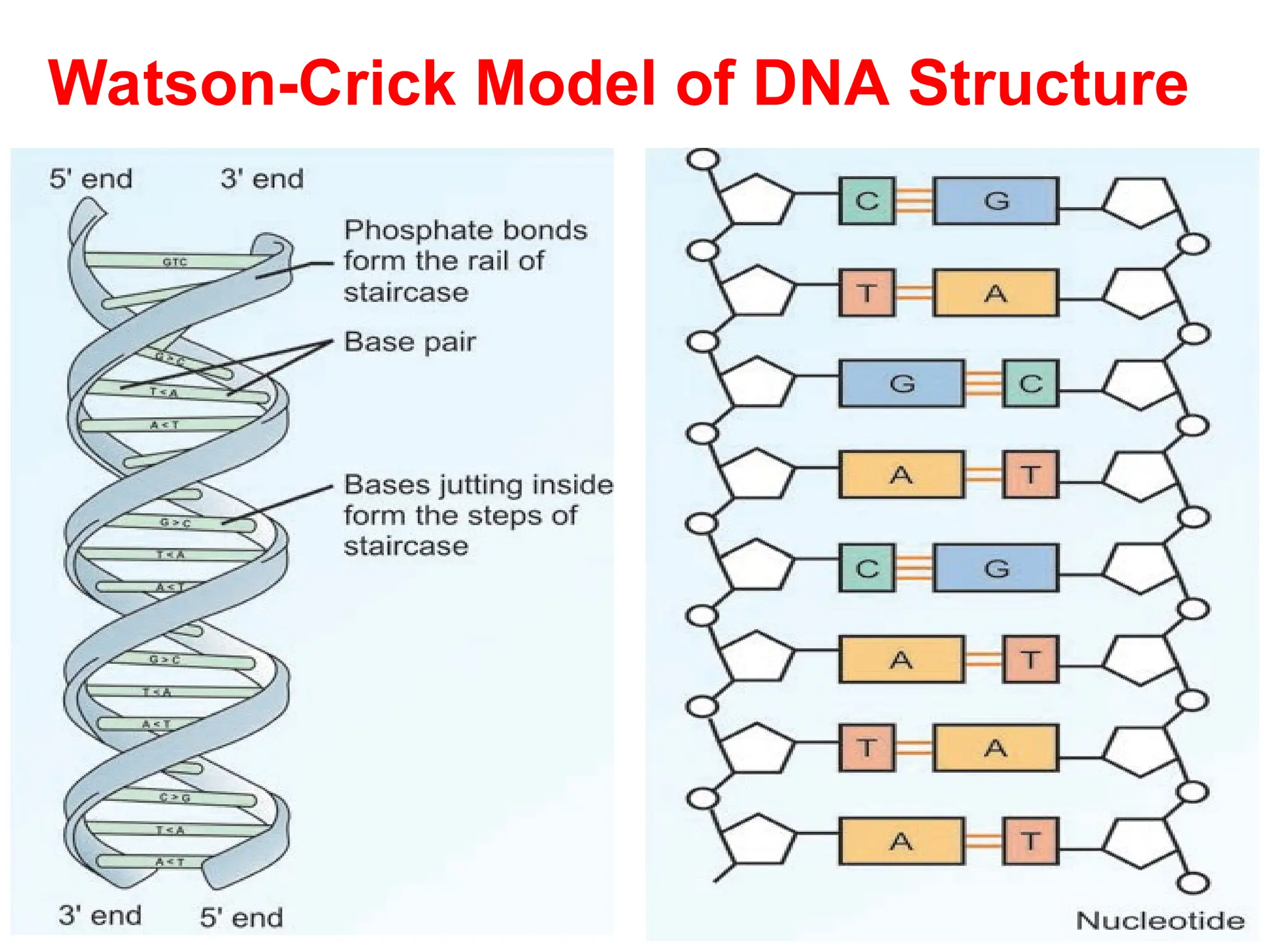
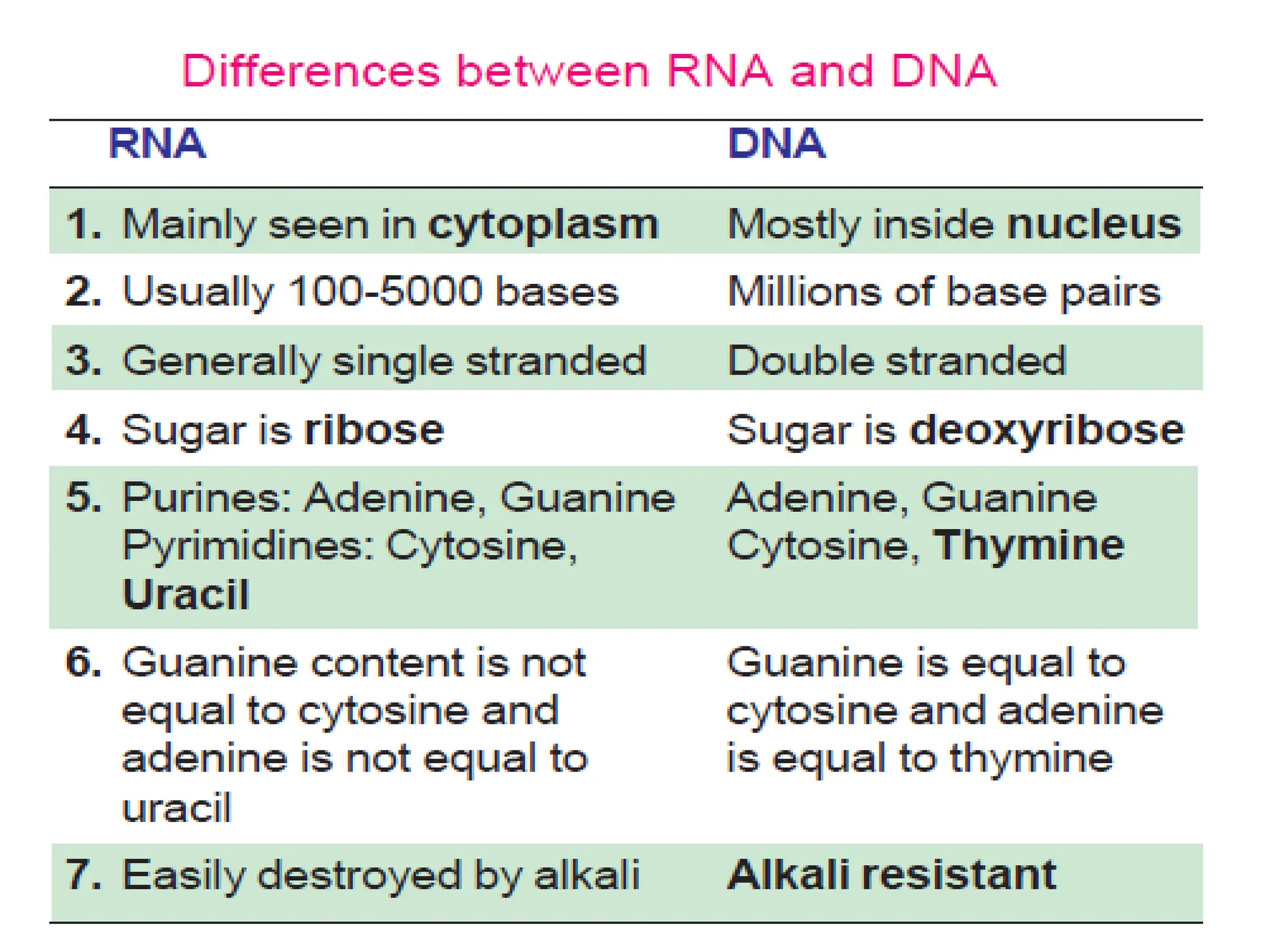
Nucleotides are the building blocks of nucleic acids, DNA and RNA, which are crucial for genetic information storage and transfer. Each nucleotide consists of a nitrogenous base, a pentose sugar, and phosphate groups, with nucleosides formed when a base attaches to sugar. The document also describes the digestion and biosynthesis of nucleic acids, their structural properties, and the roles of different types of RNA in protein synthesis.