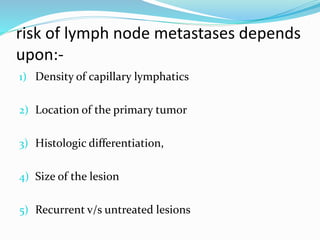
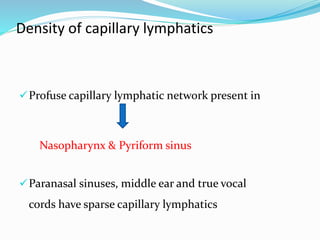
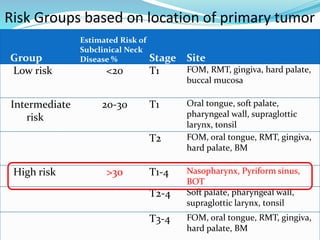
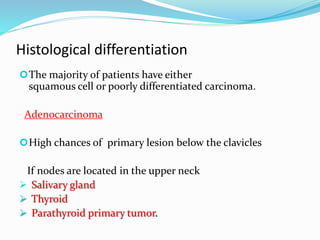
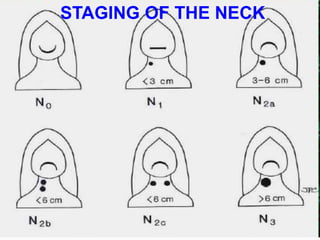
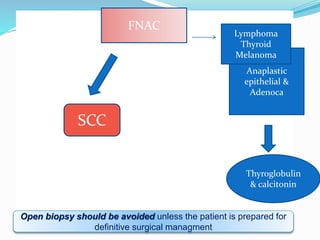
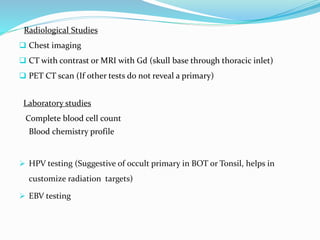
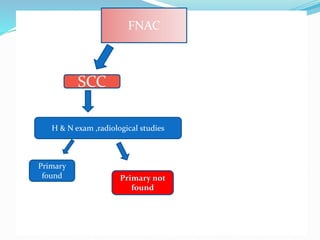
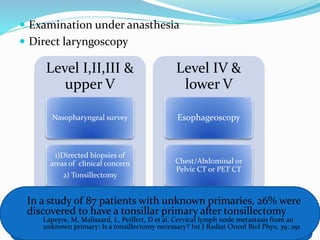
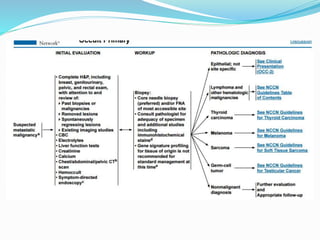
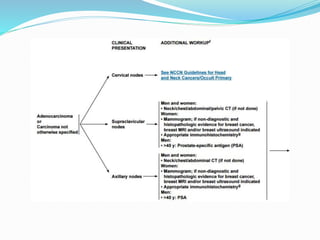
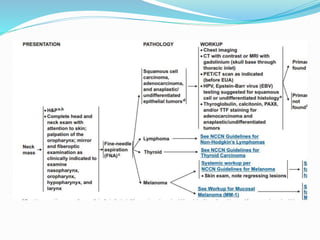
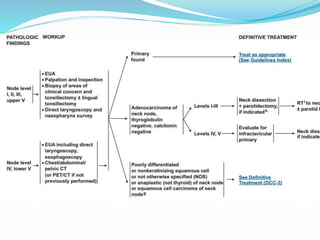
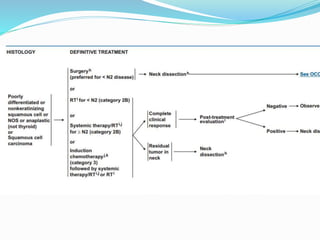
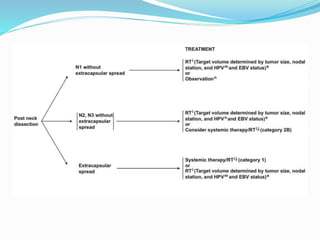
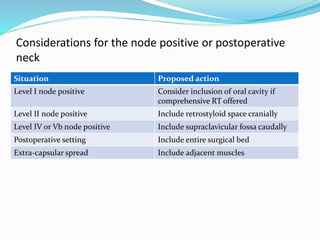
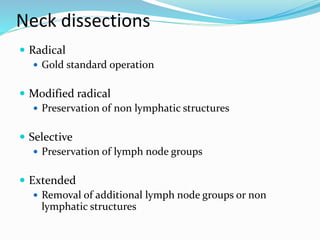
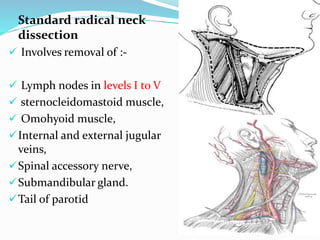
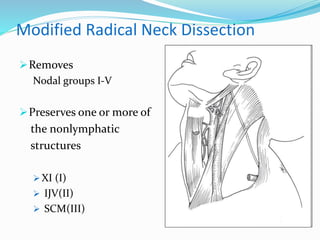
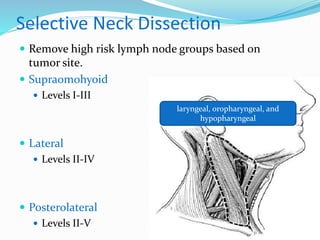
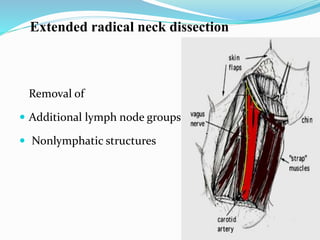
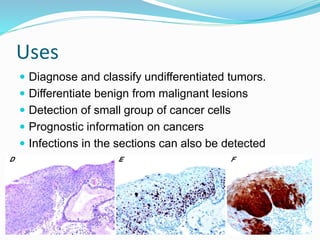
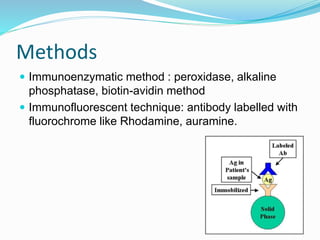
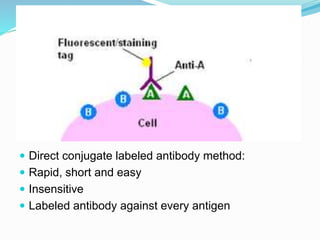
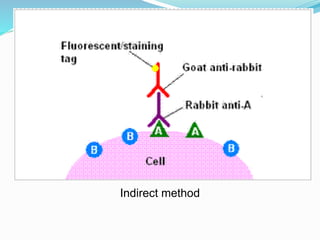
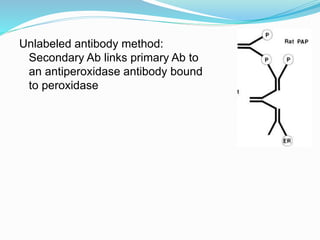
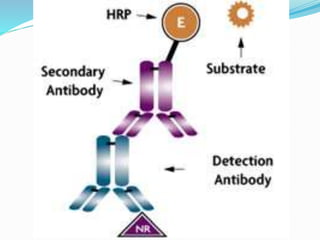
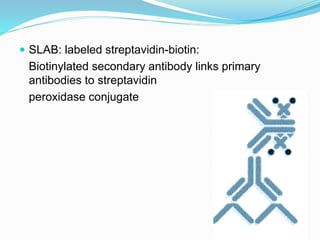
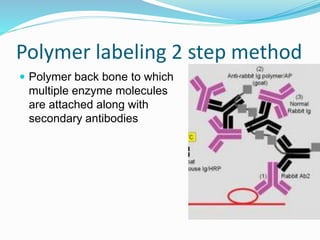
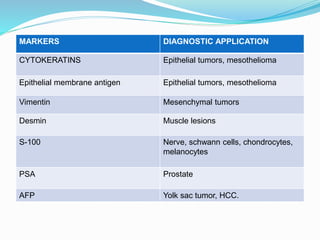
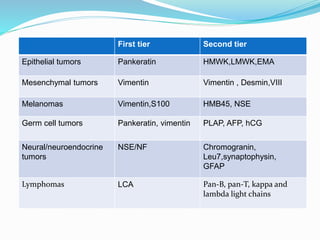
This document discusses the diagnostic and management strategies for head-and-neck carcinoma of unknown primary (HNCUP), which occurs in 3-7% of patients with metastatic squamous cell carcinoma to cervical lymph nodes. It outlines the importance of comprehensive clinical evaluations, imaging studies, and various biopsy techniques to identify potential primary lesions. Additionally, it details treatment options, including radiotherapy and surgical interventions, while highlighting the need for histological differentiation and staging considerations.