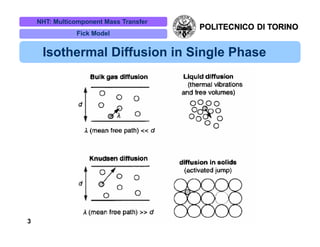
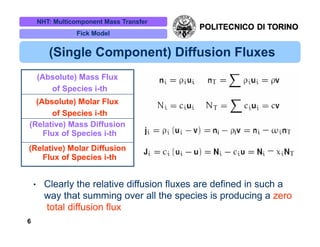
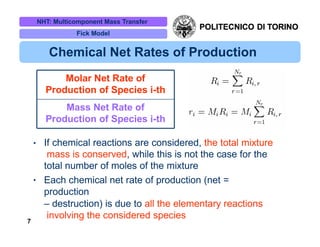
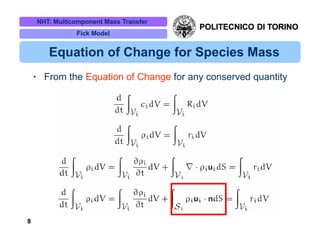
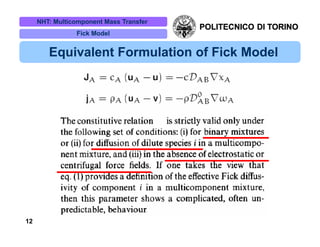
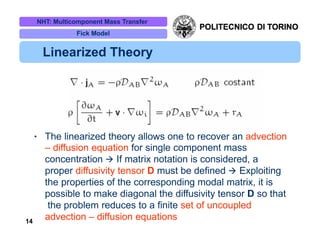
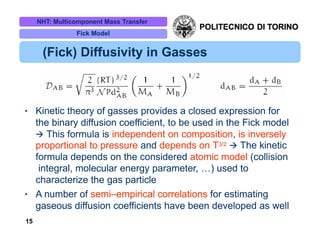
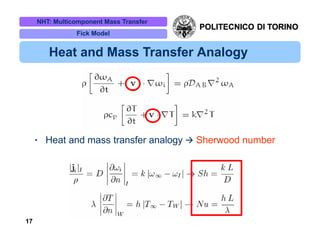
The document discusses multicomponent mass transfer, focusing on various modeling approaches such as the Fick and Maxwell-Stefan models. It addresses key concepts like diffusion fluxes, chemical net rates of production, and the analogy between heat and mass transfer, while providing insights into advanced topics like fluid flow in porous media. Additionally, it emphasizes the need for effective diffusivity methods and variations in modeling due to chemical reactions and system dynamics.