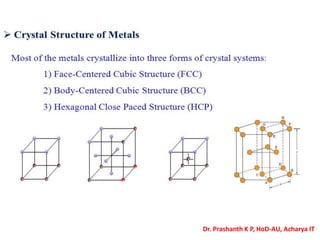
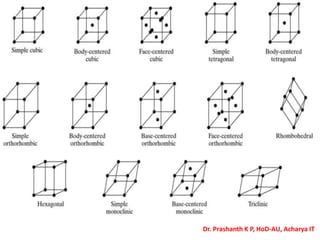
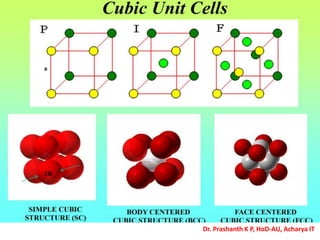
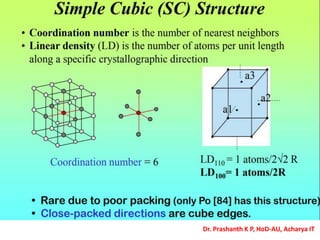
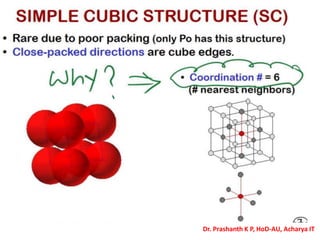
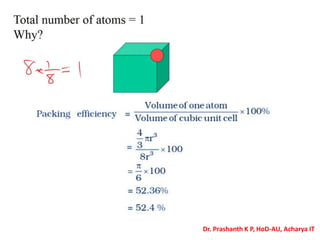
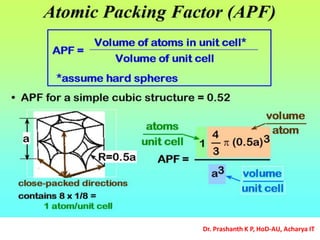
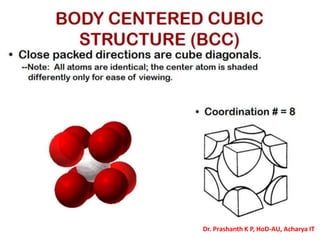
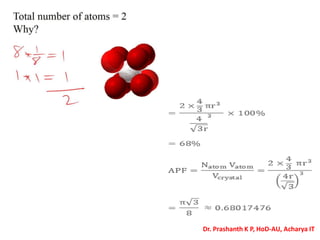
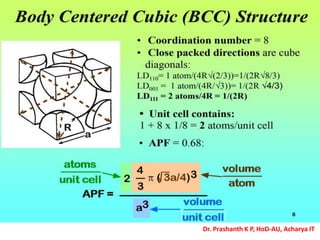
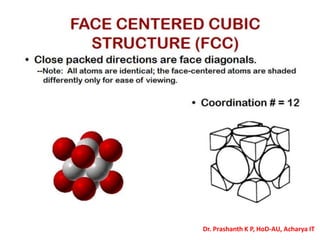
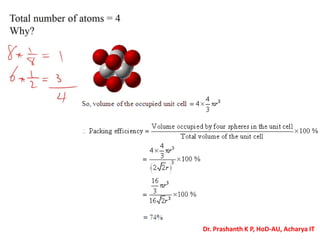
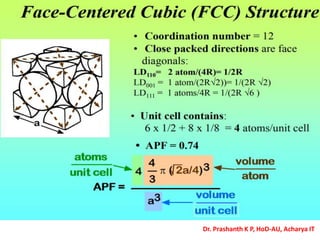
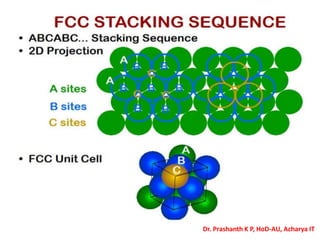
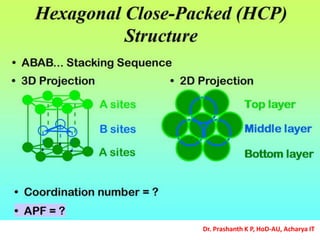
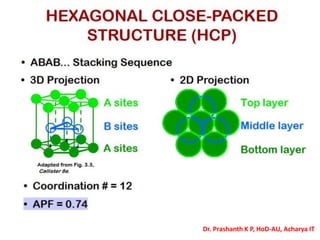
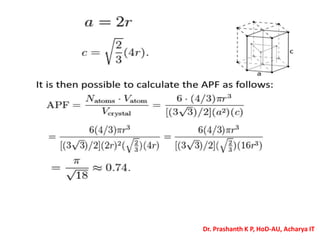
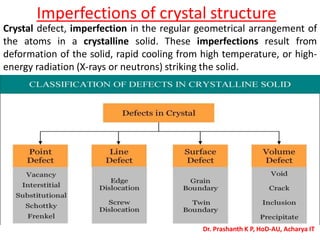
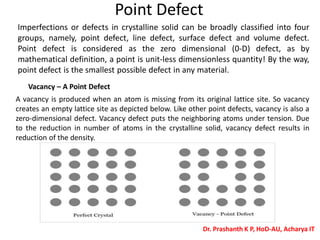
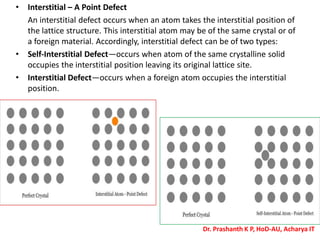
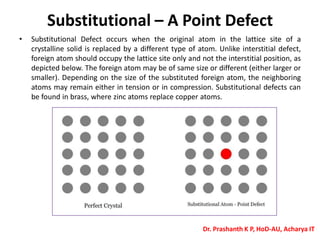
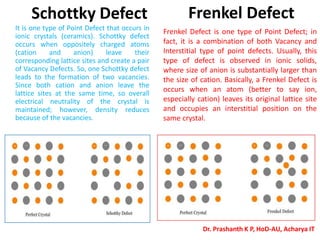
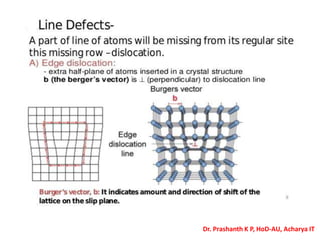
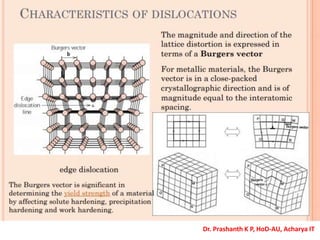
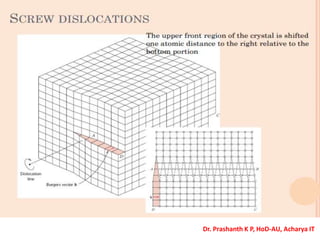
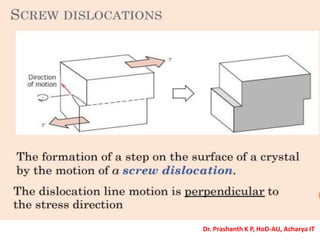
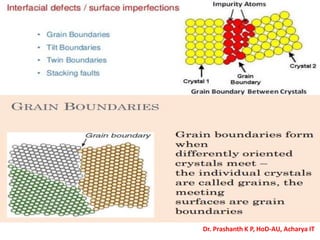
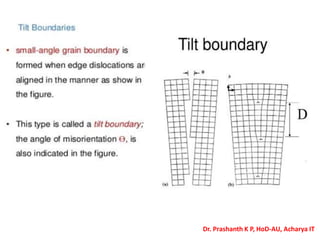
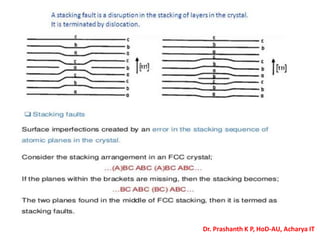
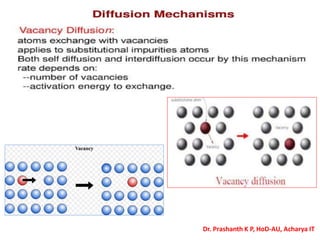
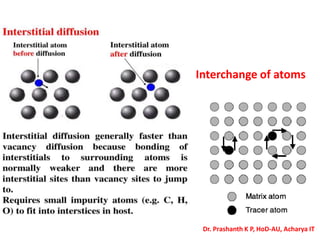
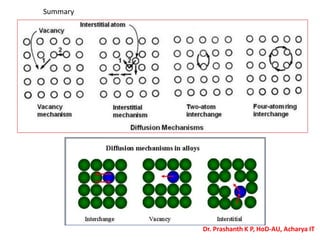
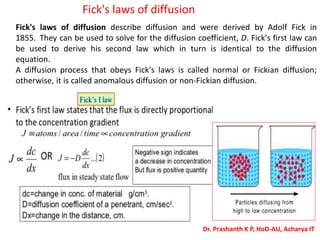
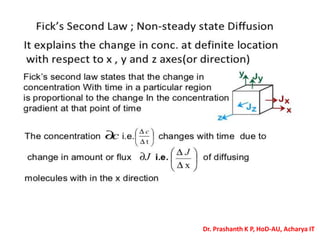
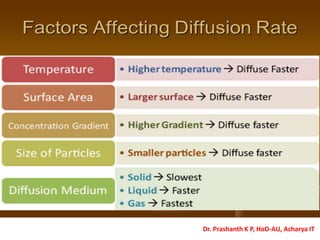
The document discusses crystal structure and defects in crystalline solids. It defines a crystal as a solid with atoms arranged in a highly ordered structure called a crystal lattice. This lattice extends in all directions and is made up of repeating patterns of units cells. Point defects are the smallest defects and include vacancies where atoms are missing, interstitials where extra atoms occupy spaces in the lattice, and substitutions where one atom replaces another. Other defect types include Schottky defects involving paired vacancies, and Frenkel defects combining a vacancy and interstitial. Atomic diffusion is discussed as the thermally activated movement of atoms, which can occur via vacancy migration, interstitial migration, or atom interchange. Fick's laws of diffusion describe