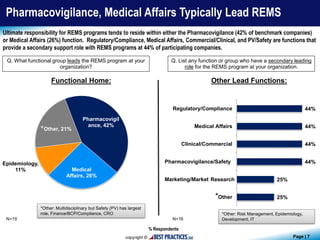
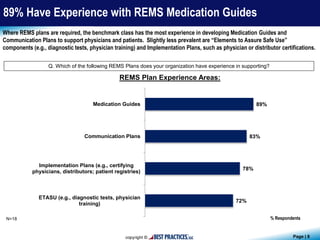
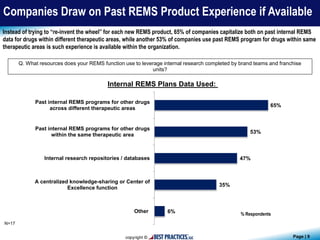
The document presents findings from a benchmark study conducted by Best Practices, LLC, analyzing how biopharmaceutical and medical device companies develop and implement Risk Evaluation and Mitigation Strategies (REMS) for newly-approved drugs. Key insights reveal that pharmacovigilance and medical affairs primarily lead REMS programs, utilizing internal databases and past program data for guidance. Additionally, the study highlights the importance of communication plans and medication guides as prevalent components of REMS plans.