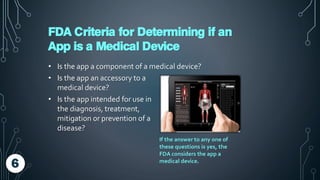
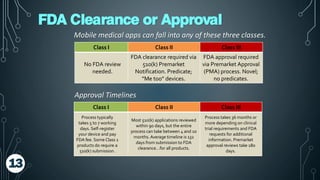
The document discusses the regulation of mobile medical apps by the FDA, outlining the criteria that determine when an app qualifies as a medical device. It explains the classification process of mobile medical apps, their categorization under FDA guidelines, and the compliance steps required for manufacturers. The summary emphasizes the evolving mobile medical app market and the importance for app developers to understand FDA regulations and processes such as the 510(k) review.