Embed presentation
Download to read offline











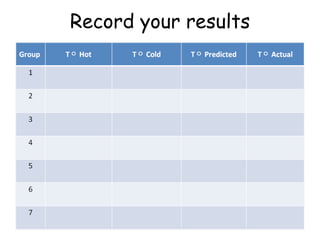

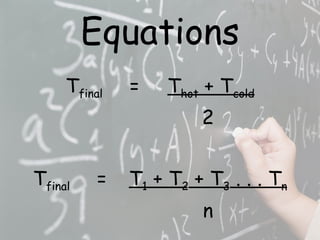

The document provides instructions for a lab experiment mixing hot and cold water. Students are asked to record their initial hypothesis about the resulting temperature and then take measurements after mixing equal amounts of 10°C and 50°C water in a third cup. They are finally asked to reflect on what happens to water particles during the mixing process.














