This document is a report submitted by Siddharth Gupta for the partial fulfillment of the requirements for a Bachelor of Technology degree in chemical engineering. The report discusses the design of a reactor for the production of polyester (PET) and compares various routes employed for the esterification of PET. It provides details on the multi-stage PET production process including the reactions, operating conditions, and products of the primary esterifier, high polymerizer, wiped film reactor, and solid state polymerization reactor. The document analyzes the advantages of direct esterification of terephthalic acid over ester interchange for PET production.






![7
Stage 1: Esterification
The major polymer backbone component called the PET (polyester) was originally developed by
Whinfield and Dickson. The initial polyester by the two was made using precursor dimethyl
terephthalate and monoethylene glycol as the raw materials [1].
Generally PET is produced by a poly-condensation reaction, using one of the following series of
chemical reaction:
Ester interchange of dimethyl terephthalate with monoethylene glycol
Direct esterification of terephthalic acid with monoethylene glycol
Reaction of ethylene oxide with terephthalic acid to form initially monomeric and low
oligomeric precursors with various monoethylene glycol to acid ratios depending
The last procedure mentioned needs an extra purification stage to extract bis-hyroxyethylene
terephthalate (BHET), hence is uneconomical. Therefore this method of route of production of
PET is generally not preferred [1].
Ester Interchange Reactions:
The method was more common due to unavailability of pure terephthalic acid.
Catalyst Preferred: salts of transitions metals, such as manganese in form of manganese octoate
(alone or in combination of divalent calcium compounds), cobalt or zinc.
The function of catalyst is to produce rapid reaction rates between the reactants at all stage of
preparation of polyester and to provide a path for reaction which produces polymer of low degree
of color.
The selection of catalyst is based on the effectiveness of the catalyst to avoid the side reactions
leading to formation of by-products of foreign matter in the polyester. This is essentially critical
with the case of those polyesters that are to be used as fibers or films as these by-products affect](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-7-2048.jpg)
![8
the main product quality. The ester interchange catalysts are potential degradants at high
temperature and hence phosphorus based stabilizers such as phosphoric acid is added prior to poly-
condensation.
The process employs ester interchange process wherein esters of di-carboxylic acid are reacted in
presence of catalyst with a glycol under conditions facilitating until removal of 99% alkanol
(generally methanol) by ester interchange with glycol and thereby forming glycol ester or low
molecular weight polymer [1].
The process initially starts in a reaction vessel equipped with a column and distillation head at the
top. The reaction vessel is heated to a temperature such that all the alkanol is allowed to pass
through the column and the glycol is retained in the column and again fed back to the reactor
vessel.
Operating Conditions: (extent of reaction 93%)
Temperature(rising profile)- from 160 degree to 230 degree
Mole ration of 2:1 for glycol to ester
By product: Methanol
Ester Interchange Reaction:
Reaction 1. Ester Interchange](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-8-2048.jpg)
![9
FIGURE 1: Block Diagram of PET Production
Direct Esterification Reactions:
This process is more commonly used in process industries due to availability of pure terephthalic
acid.
Catalysts Preferred: generally no catalyst are used but alkaoxide compounds of metals such as
titanium, tin and antimony can be used as catalysts.
For Commercial purposes, it is required that polyester resins be produced in shortest possible time
and desired degree of polymerization, physical and chemical properties be attained. Since,
solubility of terephthalic acid in glycol is low, therefore in absence of a catalyst the reaction is
carried out at elevated temperatures.
The high acidity of terephthalic acid catalyzes the formation of di-ethylene glycol, hence must be
reduced appreciably alkali, alkaline and quaternary ammonium compounds to obtain the desired
polymeric properties [3].](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-9-2048.jpg)
![10
The process is generally carried out in at elevated temperatures in absence of oxygen gas at
atmospheric or elevated pressures for 2 to 4 hours. When a clear mixture is obtained or process is
near completion or completed any leftover glycol can be distilled off and poly-condensation
catalysts can be added.
There are two major steps for production of PTA:
1. Catalytic Oxidation of PX to Make Crude Terephthalic Acid (CTA): This involves
oxidation, Crystallisation, solvent recovery, filtering, drying etc.
2. Purification of CTA to make PTA: Involves hydrogenation, crystallization (Amco process),
Centrifuging, drying, conveying, storage, bagging etc., or by leaching and sublimation
(Mobil Process) [5].
Operating Conditions: (extent of reaction 93%-94%)
Temperature- 230 degree c to 290 degree c
Mole ratio of 1.2-1.6 for glycol to ester
Pressure of reactor- atmospheric or above
By Product: Water
Reaction 2. Direct Esterification](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-10-2048.jpg)
![11
Some advantages of direct esterification over ester interchange are as follows: [3]
•Per unit of polyester produced – 15% less TPA is required
•Bulk density of TPA is 1.0 tons/m3 as compared to DMT (0.5 tons/m3). Thus transportation costs
and storage requirements for TPA are significantly lower.
•TPA process required a lesser feed mole ratio of glycol to PTA of around 1.2-1.6 against 2 for
DMT
•Esterification reaction of TPA does not require any catalyst whereas the trans- esterification of
DMT has to be catalyzed.
•With TPA it is simpler to maintain a constant degree of esterification. In case of DMT trans-
esterification step is very sensitive to the quality of raw material, changes in instantaneous mole
ratio and etc.
• With TPA process water is the By-product whereas with DMT process methanol is the By-
product. Therefore, more process hazards in handling methanol.
•Product from TPA is better with respect to thermal and hydrolytic stability.](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-11-2048.jpg)
![12
Figure 2: Block Diagram
Stage 2: POLYCONDENSATION REACTION
The second step in PET synthesis is similar for both the ester interchange and direct esterification
routes. A further catalyst is to be added to the mixture of linear oligomers, free glycol is distilled
out according to reaction [6].](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-12-2048.jpg)
![13
and the temperature is raised until it reaches 260-290. The pressure is reduced as rapidly as possible
without causing carry-over due to foaming and polymerization is carried out finally at a pressure
below 1 mm Hg until the required molecular
The most popular catalyst for this stage is antimony trioxide, although antimony pentoxide and
germanium dioxide has also been used
The poly-condensation reaction is reversible as it is shown in the above reaction so it is important
to remove the glycol produced from the system as quickly as possible. In most polymerization
reactors, the rate of attainment of high molecular weight is diffusion-controlled rather than
chemically controlled, and it is useful to minimize the diffusion path by maximizing the surface:
volume ratio and the rate of generation of free surface [7].
There are two types of poly-condensation, the melt and the solid poly-condensation. The melt poly-
condensation of PET, carried under common industrial conditions, leads to products with a
molecular weight of 15,000-25,000. Certain injection molding and extrusion applications require
high quality, high molecular weight polyesters with improved mechanical and thermal properties.
PET with a molecular weight of more than 30,000 can rarely be prepared under conditions of melt
polymerization because of the long reaction times and because at high poly-condensation
temperature, thermal degradation occurs more rapidly than formation. Higher molecular weight
PET can be obtained more easily by poly-condensation in the solid phase below the PET‘s melt
temperature because at the lower temperatures, the speed of formation is higher than the speed of
degradation.
A major side reaction that can take place in addition to the previous main reactions is the
Formation of diethylene glycol (DEG). The formation of this side product is important in preparing
PET. The amount of DEG in PET molecules influences many important properties of the polymer
[2].](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-13-2048.jpg)
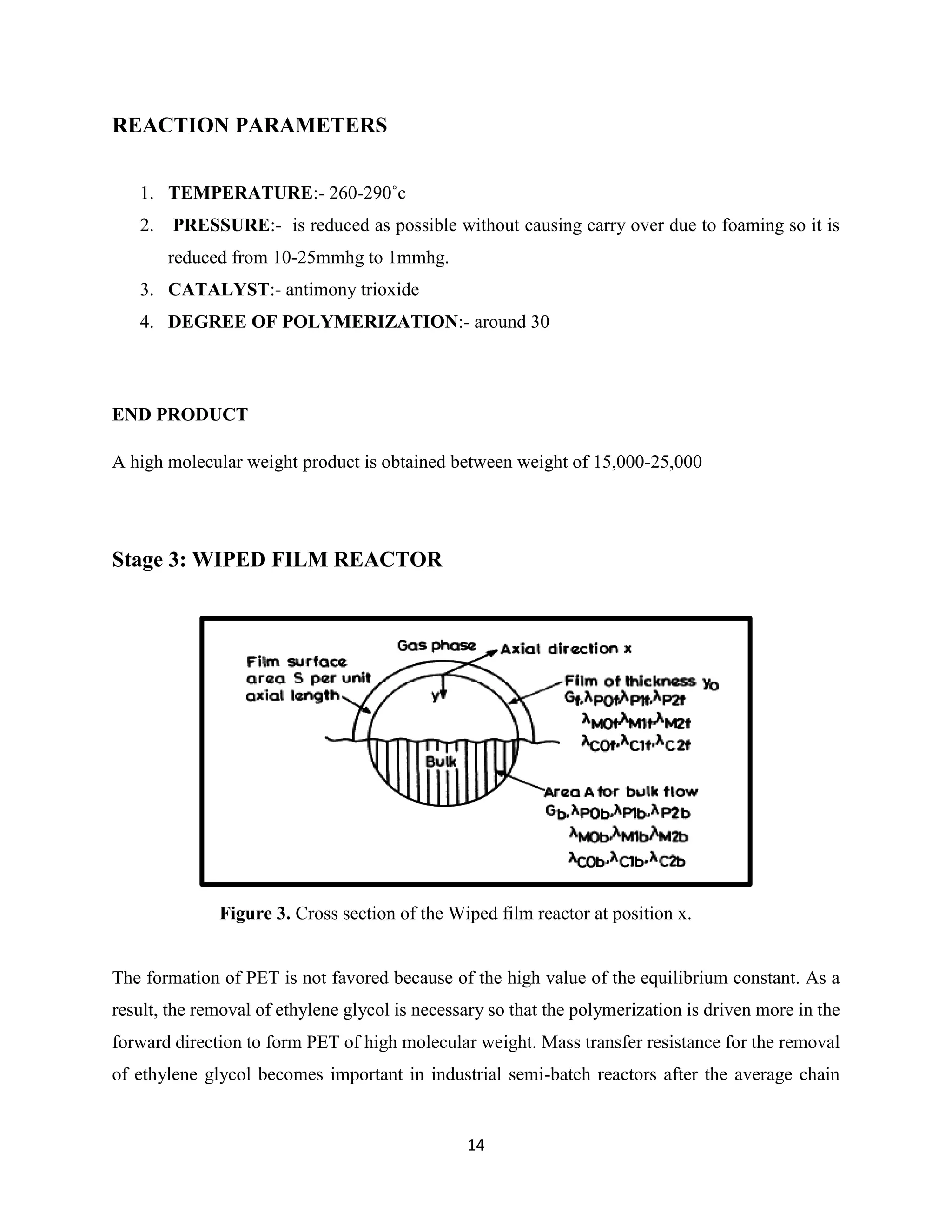
![15
length increases beyond 30, and the polymerization is then usually carried out in special wiped
film reactors [7].
There are several geometries of the wiped film reactor. A schematic diagram of a reactor
commonly employed industrially for PET is shown in Figure. The reactor is designed in such a
way in order to provide a high surface area of reaction for Mass Transfer to take place and also to
provide the desired flow pattern. The bulk of the polymer flows in the axial direction, from which
some of the polymer is spread on the cylindrical wall as a thin film. This film is scraped after a
certain exposure time and mixed with the bulk of the polymer fluid. Fluid transport in spreading
and scraping the film has been analyzed by McKelvey, who found that there is a bow wave
formation on the edge of the blade. Fluid motion within the bow wave on the blade tends to mix
the fluid within it and therefore each of the blades serves as a mixer. The axial transport of the
fluid is assisted by the use of blades, ribbons, screws, etc., and in designing these, it is desired to
minimize axial mixing but simultaneously having infinite radial mixing.
In the analysis of wiped film reactors, it is assumed that there is no axial mixing but there is infinite
radial mixing. It is also assumed that a small amount of polymeric material of the bulk is spread
as a film, so that its mixing (after it is scraped) with the bulk contributes negligibly to the change
in molecular weight of the polymer [6].
OPERATING CONDITIONS:
Temperature : 540-574 kelvin
Pressure : 5 milli-bar
END PRODUCT:
A Polymer of high Molecular Weight around 15000-20000 is attained
A very high viscous Polymer product is obtained](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-15-2048.jpg)
![16
Stage 4: Solid State Polymerization
After polymerization in the melt phase, the molecular weight of polyester can be further increased
by poly-condensation of PET chips in the solid state and this process is called solid state
polymerization (SSP). This process enables higher molecular weights to be reached which are
either technically or commercially not feasible in the melt phase.
it was recognized that it is economically more advantageous to complete the polymerization of
PET by a "solid stating" process than to continue the melt polymerization for a substantial extra
number of hours in order to bring the intrinsic viscosity of the final product from about 0.7 dL/g
to around 1.0 dL/g.
Disadvantages of latter stage of PET polymerization (usually carried out in the finishers): [8]
time-consuming,
prone to cause oxidative degradation and yellowing,
is the slowest step in the polymerization train,
Therefore, we replace the latter step by solid stating, which is carried out separately, because
generally increases the throughput of the polymerization train
and minimizes the degradation and discoloration of high-MW PET.](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-16-2048.jpg)
![17
Figure 4: Outline of the solid-state polymerization reactor
Source: Handbook of Thermoplastic Polymers: Homopolymers, Copolymers, Blends, and
Composites.
The SSP step (Figure 1) follows the melt polymerization mechanism, and is used to upgrade the
"low I.V. resin" and reduce the AA content. Here, "low I.V." means ~ 0.6 dL/g. The melt
polymerization reactor product which has a low I.V. and high AA-containing amorphous chips
cannot be fed directly to the SSP reactor, operating at 200-220 degree C, because the amorphous
chips would stick and form agglomerates if heated suddenly above the Tg [8].
Therefore, before the SSP reactor, there are two units to crystallize the chips.](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-17-2048.jpg)
![18
Firstly, the amorphous chips enter a fluidized pre-crystallizer where they are crystallized at ~170
degree C with vigorous agitation to prevent sticking. The amorphous transparent chips turn
opaque because of the formation of spherulites.
The chips are further crystallized at higher temperature ranges (~ 190 degree C) in a rotary
crystallizer, which consists of a heated vessel with a rotating stirring element (a screw or a shaft
with paddles) which pushes the chips forward. Air or nitrogen may be used during the
crystallization [4].
The chips leaving the rotary crystallizer have a density of 1.3845g/cubic cm (having a crystallinity
of 30%). Therefore an elemental understanding of PET crystallization is very important and
advisable for the design of the crystallizers.
Figure 5: Outline of the solid-state polymerization reactor
Source: Handbook of Thermoplastic Polymers: Homopolymers, Copolymers, Blends, and
Composites. Ed. Stoyko Fakirov](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-18-2048.jpg)
![19
The moving bed, or gravity-flow continuous SSP reactor,(Figure 2) essentially consists of a long
tube where a bed or column of polymerizing chips moves by gravity downwards while an inert gas
is passed through the moving bed in a counter-current direction at a velocity which is insufficient
to raise or fluidize the bed but sufficient to remove the volatile reaction products such as EG and
AA. The height of the moving resin column is maintained essentially constant by the continuous
addition of the crystallized low I.V. resin at the top of the column and withdrawal of the high I.V.
material at the bottom [8].
The reactor aims to provide plug flow so that the chips experience close to uniform residence time.
However in practice, there is a distribution of residence times, and hence the SSP chips generally
show greater I.V. variation than that of the melt-polymerized material. The gas-to-solids ratio in
the reactor and the temperature setting are used to control the rate of the reaction. In the SSP
reactor, not only does polymerization take place, but so also does additional crystallization. The
final chips reach an I.V. of 0.70-0.85dL/g, depending on the intended application, and a density of
~ 1.400 g/cubic cm [4].](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-19-2048.jpg)
![20
Figure 6: "Solid stating" of PET.
V-IOl A&B: Pellet surge bins; M-IOl A&B: Pellet screw feeders; S-IOl A&B: PET crystallizers;
S-103 A&B: Hopper driers; S-104 A&B: PET preheaters; S-105 A&B: Purge vessels; R-IOl
A&B: Hopper reactors; S-107 A&B: PET product coolers; V-102 A&B: Pellet hoppers; V-103
A: PET product storage silo; CW = cold water
Source: Industrial-Scale Production of Polyesters, Especially Poly(ethylene terephthalate), S. M.
Aharoni
After exit from the SSP reactor, the polymer is sent to a processing unit (Figure: 3) where the chips
are cooled and simultaneously the fine PET dust (arising from friction between chips) is separated
by a cyclone separator. SSP takes place faster in the PET dust, resulting in a much higher I.V.
(1.0dL/g) than that in the chips. This high I.V. PET powder can pose rheological problems during
injection molding, and hence its removal is very important. Because PET is extremely sensitive to
the presence in it of very low levels of moisture, it is advisable to keep the PET product of the
solid stating process bone-dry, and/or to re-dry the pellets to 10 ppm or less water prior to their
use in any forming or shaping process in which they are melted [8].](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-20-2048.jpg)
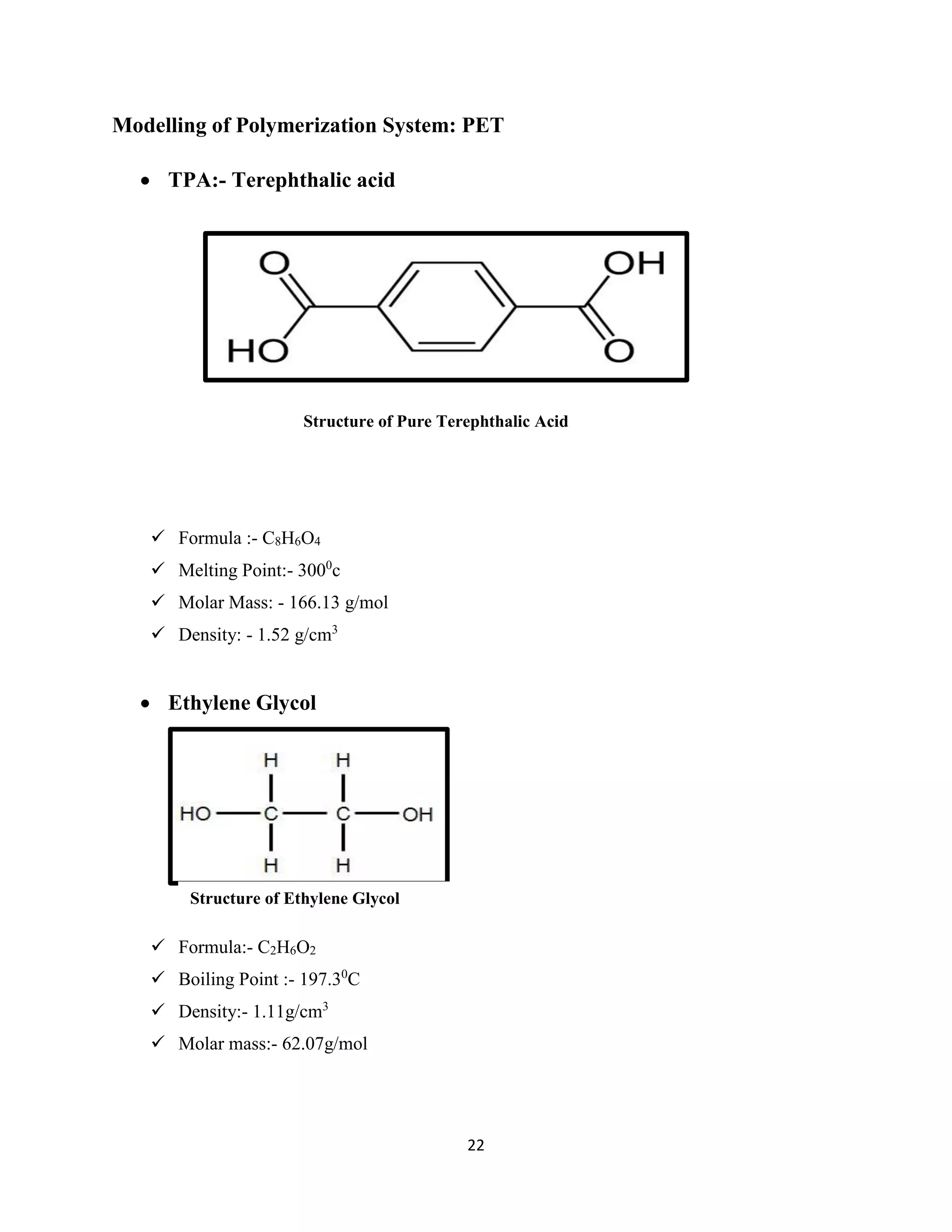
![21
Modelling of Polymerization systems: A-B System
Consider the set of Following Reactions: (Series and Parallel)
A-B + A-B A-(BA)-B
A-(BA)-B + A-B A-(BA-BA)-B
General Equation can be written as:
Pn + Pm P n+m
Note: Due to large no. equilibrium constants involved is it considered using ‘Flory equal
Reactivity Hypothesis’ that Knm = K
Hence General Equation turn out to be:
d[pn]/dt = -2KPnP + K ∑ Pr Pn-r
K
K
R=1
∞
K](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-21-2048.jpg)

![24
NOTE: - The reactions are different but rate constant is K from Flory Hypothesis
MOLE BALANCE EQUATIONS
dP1/dt = -4KP1Q1 – 2KP1R1 -2KR2P1 – 2KR3P……..
= -2K { 2P1Q1 + P1 [ R1+ R2+R3-----]}
o Let R1+R2+R3 +------ +Rn= R
So,
dP1/dt = -2 K P1 [2Q1+R]
dP1/dt = -4KP1Q1 – 2KP1R
Similarly
dP2/dt = 2 K P1 R1 – 4 K P2 Q1 – 2 K P2 R
dP3/dt = 2 K P1 R2 + 2 K R1 P2 – 4 P3 Q1- 2 K P3 R
On generalizing, we get
dPn/dt = 2K ∑∞
𝑛=1 ∑ 𝑛−1
𝑟=1 [Pr] [Rn-r] - 4 K Pn Q1 – 2 K Pn R](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-24-2048.jpg)
![25
Upon following the same procedure, we get :
d[Q1]/dT = -4K P1 Q1 – 2 K Q1 R
d[Q2]/dT = 2 K [Q1] [R1] – 4 KQ2 P1-2 K Q2 R
d[Q3]/dT = 2K Q2 R1 + 2 K R2 Q1 - 4K Q3 P1 – 2 KQ3 R
Upon generalizing, we get :
d[Qn]/dt = 2 K ∑∞
𝒏=𝟏 ∑ 𝒏−𝟏
𝒓=𝟏 QrRn-r- 4 K Qn P1 – 2 K Qn R
Similarly, we can obtain the following :
dRn/dt = 4 K Pn Q1 + 2 K ∑∞
𝒏=𝟏 ∑ 𝒏−𝟏
𝒓=𝟏 Rr Rn-r + 2 K Rn [ P + Q + R ] + 4 K Qn P
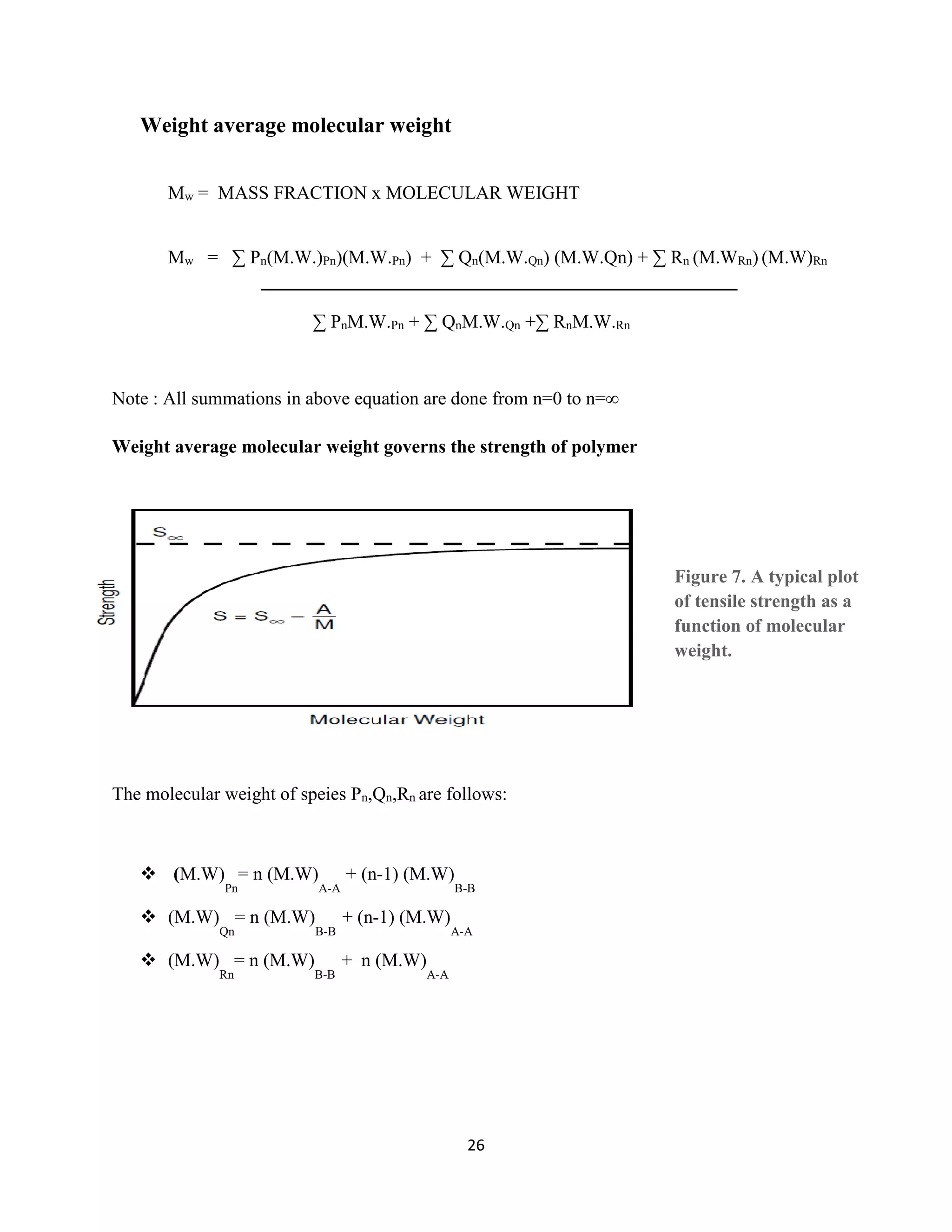
Number average molecular weight
Mn=
𝑇𝑂𝑇𝐴𝐿 𝑀𝐴𝑆𝑆
𝑇𝑂𝑇𝐴𝐿 𝑀𝑂𝐿𝐸𝑆
Therefore,
Mn=
∑𝐏𝐧(𝐌.𝐖.𝐏𝐧)
∑(𝐏𝐧+𝐐𝐧+𝐑𝐧
+
∑𝐐𝐧(𝐌.𝐖)𝐐𝐧
∑𝐏𝐧+𝐐𝐧+𝐑𝐧)
+
∑𝐑𝐧(𝐌.𝐖)𝐑𝐧
𝐏𝐧+𝐐𝐧+𝐑𝐧
The number average molecular weight of polymer determines the colligative properties like:-
RELATIVE LOWERING OF VAPOUR PRESSURE
ELEVATION OF BOILING POINT
DEPRESSION OF FREEZING POINT
OSMOTIC PRESSURE](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-25-2048.jpg)
![27
Calculation Of Number Average Molecular Weight :
As derived earlier ;
dP1/dt = -4KP1Q1 – 2KP1R
So,
d[P1.(M.WP1)]/dt = [-4KP1Q1 – 2KP1R]( M.WP1)
Putting value of (M.WP1)
d[P1.(M.WP1)/dt = [-4KP1Q1 – 2KP1R]( M.WA-A)
Similarly:
d[P2(M.WP2)]/dt = [ 2 K P1 R1 – 4 K P2 Q1 – 2 K P2 R][ 2M.WA-A+ M.WB-B]
Generalizing
d[Pn(M.WPn)]/dt =[ 2K ∑∞
𝑛=1 ∑ 𝑛−1
𝑟=1 [Pr][Rn-r]-4 K Pn Q1 –2 K Pn R][n M.WA-A+(n-1)M.WB-B]
Adding L.H.S & R.H.S, we get :
d[P1(M.WP1)+.....+ Pn (M.WPn)]/dt = [[-4KP1Q1 – 2KP1R]( M.WA-A)+[ 2K ∑∞
𝑛=1 ∑ 𝑛−1
𝑟=1
[Pr] [Rn-r] - 4 K Pn Q1 – 2 K Pn R][n M.WA-A+ (n-1)M.WB-B ]
So, generalizing we get;
d[∑ Pn(M.WPn)]/dt = -4KQ1[∑∞
𝑛=1 nPn( M.WA-A) + ∑∞
𝑛=1 (n-1)Pn( M.WB-B)] – 2KR
[∑∞
𝑛=1 nPn( M.WA-A) + ∑∞
𝑛=1 (n-1)Pn( M.WB-B)] + 2K[∑∞
𝑛=1 ∑∞
𝑟=1 (r+n) PnPr(M.WA-A) +
∑∞
𝑛=1 ∑∞
𝑟=1 (r+n-1)Pn-1Rr ( M.WB-B)]](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-27-2048.jpg)
![28
Solving this;
d[∑ Pn(M.WPn)]/dt = -4KQ1[∑∞
𝑛=1 nPn( M.WA-A+ M.WB-B) - ∑∞
𝑛=1 Pn( M.WB-B)]
-2KR[∑∞
𝑛=1 nPn( M.WA-A+ M.WB-B) - ∑∞
𝑛=1 Pn( M.WB-B)]+ 2K[∑∞
𝑛=1 ∑∞
𝑟=1 (r+n)
PnPr(M.WA-A+ M.WB-B) + ∑∞
𝑛=1 ∑∞
𝑟=1 PnRr (M.WB-B)]
So,
d[∑ Pn(M.WPn)]/dt = -4KQ1[(∑∞
𝒏=𝟏 nPn)M.WR1 – [P]M.WB-B] -2KR [(∑∞
𝒏=𝟏 nPn)M.WR1 – [P]M.WB-B] +
2K[(∑∞
𝒏=𝟏 ∑∞
𝒓=𝟏 (r+n) PnPr)M.WR1 + ∑∞
𝒏=𝟏 ∑∞
𝒓=𝟏 PnRr(M.WA-A) ]
Following the same procedure for [Q], we get:
d[∑ Qn(M.WQn)]/dt = -4KP1[(∑∞
𝒏=𝟏 nQn)M.WR1 –[Q]M.WB-B] -2KR[(∑∞
𝒏=𝟏 nQn)M.WR1 –[Q]M.WB-B] +
2K[(∑∞
𝒏=𝟏 ∑∞
𝒓=𝟏 (r+n) QnRr)M.WR1 + ∑∞
𝒏=𝟏 ∑∞
𝒓=𝟏 QnRr(M.WA-A) ]
Following the same procedure for [R], we get;
d[∑ Rn(M.WRn)]/dt = 4KQ1[(∑∞
𝒏=𝟏 nPn) - 2K[P + Q + R][(∑∞
𝒏=𝟏 nRn) ]] + [2K[(∑∞
𝒏=𝟏 ∑∞
𝒓=𝟏 (r+n) RnRr)]
+ 4KP1[ ∑∞
𝒏=𝟏 𝒏Qn]](M.WR1)
Calculation for expression d∑ Pn/dt, d∑ Qn/dt, d∑ Rn/dt
dP1/dt = -4KP1Q1 – 2KP1R
dP2/dt = 2 K P1 R1 – 4 K P2 Q1 – 2 K P2 R
Generalizing:
dPn/dt = 2K ∑∞
𝑛=1 ∑ 𝑛−1
𝑟=1 [Pr] [Rn-r] - 4 K Pn Q1 – 2 K Pn R](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-28-2048.jpg)
![29
Adding LHS & RHS & Solving;
d∑ Pn/dt = – 2K[P]R1-4K[P]Q1 + 2K[P][R] - equation(1)
Similarly;
d∑ Qn/dt = – 2K[Q]R - 4KP1[Q] + 2K[Q][R] - equation(2)
d∑ Rn/dt = 4KPQ1 + 2KPR + 2KQR + 2KQP1 - equation(3)
Adding Equations 1, 2, 3;
d(∑ Pn+Qn+Rn)/dt = 2KPR + 2KQR
So,
d(∑ Pn+Qn+Rn)/dt = 2KPR + 2KQR - equation(4)
Now, let us define moments to be :-
λ0,1,2 = ∑∞
𝐧=𝟏 n0,1,2 [Pn]
μ0,1,2 = ∑∞
𝐧=𝟏 n0,1,2 [Qn]
ϕ0,1,2 = ∑∞
𝐧=𝟏 n0,1,2 [Rn]
Converting the above derived equation in terms of moments, we get ;
d[∑ Pn(M.WPn)]/dt = 2K[(λ0 ϕ1 – 2Q1 λ1) M.WR1 + 2Q1λ0(M.WB-B)] - equation(5)
Similarly :-
d[∑ Qn(M.WQn)]/dt = 2K[μ0ϕ1 – 2P1μ1] M.WR1 + 2P1μ 0(M.WA-A)] - equation(6)
Similarly :-
d[∑ Rn(M.WRn)]/dt = 2K[2Q1λ1 - (λ0 + μ0 + ϕ0) ϕ1 + 2P1μ1 + 2 ϕ0ϕ1] M.WR1 -equation(7)](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-29-2048.jpg)
![30
Adding Equations 5, 6, 7, we get ;
d[∑ Pn(M.WPn)+Qn(M.WQn)+Rn(M.WRn)]/dt= [ϕ0ϕ1(M.WA-A+M.WB-B)+2P1μ 0(M.WA-A)+2Q1λ0(M.WB-B)]
equation(8)
Dividing Equation 8 from Equation 4, we get ;
d[(∑ Pn(M.WPn)+Qn(M.WQn)+Rn(M.WRn))/(∑ Pn+Qn+Rn)]=[(ϕ0ϕ1+2P1μ0)M.WA-A+(ϕ0ϕ1+
2Q1λ0)M.WB-B]/[( λ0 + μ0) ϕ0] + [(ϕ0ϕ1 + 2P1μ0) /( λ0 + μ0) ϕ0] M.WA-A + [(ϕ0ϕ1 + 2Q1λ0)/( λ0 + μ0)
ϕ0] M.WB-B
So,
d(∑ Pn(M.WPn)+Qn(M.WQn)+Rn(M.WRn))/d(∑ Pn+Qn+Rn)=[(ϕ0ϕ1+2P1μ0)/(λ0+μ0)ϕ0] M.WA-A +
[(ϕ0ϕ1 + 2Q1λ0)/( λ0 + μ0) ϕ0] M.WB-B
General reactor balance equations:
Product
P1,out = P1 + P2 + . . . .
Q1,out = Q1 + Q2 + . . .
R1,out = R1 + R2 + . . .](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-30-2048.jpg)


![33
Where:
𝑅 𝑒𝑣𝑎𝑝 = 𝑅𝑎𝑡𝑒 of evaporation = Kp (A) [PS - PR]
PS =Partial Pressure of EG on surface of liquid
PR = Reactor operating pressure
Kp = Mass transfer coefficient
Mole balance of R :
𝑅1𝑖𝑛 = 𝑅1 +
{4 𝐾 𝑃1 𝑄1 − 2 𝐾 𝑅1 (𝑃 + 𝑄 + 𝑅) + 4𝐾 𝑄1 𝑃1} . 𝑣
Q° 𝐹
𝑅 𝑛,𝑖𝑛
= 𝑅 𝑛
+
{4 𝐾 𝑃𝑛 𝑄1 + 2 𝐾 ( 𝑅 𝑛−1 𝑅1 + 𝑅 𝑛−2 𝑅2 + ⋯ ) − 2𝐾𝑅 𝑛( 𝑃 + 𝑄 + 𝑅) + 4𝐾𝑄 𝑛 𝑃1 } . 𝑣
Q° 𝐹
Adding LHS and RHS and converting it in terms of moments, we get:
0 = 𝜙0 + {4𝐾𝑄1 𝜆0 + 4𝐾𝑃1µ0 − 2𝐾𝜙0 ( 𝜆0 + µ0 + 𝜙0) + 2𝐾𝜙2 𝜙1}.
𝑣
Q° 𝐹
Therefore, we get :
𝜙0 + [4𝐾𝑄1 𝜆0 + 4𝐾𝑃1µ0 − 2𝐾𝜙0( 𝜙0 + µ0 + 𝜆0) + 2𝐾𝜙0 𝜙1]
𝑣
Q° 𝐹
= 0](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-33-2048.jpg)
![34
Reactor Balance Equation for BHET (Q2) :
𝑄2,𝑖𝑛 = 𝑄2,𝑜𝑢𝑡 + {2𝐾 [ 𝑄1] [ 𝑅1] − 4𝐾𝑄2 𝑃1 − 2𝐾𝑄2 𝑅}.
𝑣
Q° 𝐹
𝑄2,𝑖𝑛 = 𝑄2 +
(2𝐾𝑄1 𝑅1 − 4𝐾𝑄2 𝑃1 − 2𝐾𝑄2 𝑅). 𝑣
Q° 𝐹
Hence, we get :
𝑄2 ( 𝐵𝐻𝐸𝑇) =
𝑄2,𝑖𝑛 −
(2𝐾𝑄1 𝑅1). 𝑣
Q° 𝐹
{1 − 4𝐾𝑃1
𝑣
Q° 𝐹
− 2𝐾𝑅
𝑣
Q° 𝐹
}
Properties of BHET: [6]
Note: The above reactor balance equations are non-linear algebraic equations and can be solved
using multi-variable Newton-Raphson Technique.
Density (1.3 ± .1) g/cm3
Boiling Point 446.5 ± 32 0
C at 760 mm Hg pressure
Vapour Pressure 0.0 ± 1.1 mm Hg at 25 0
C
Index of Refraction 1.556](https://image.slidesharecdn.com/fff3c227-3bca-410e-b7a4-b4594c1a7381-150623080959-lva1-app6892/75/Minor-Project-34-2048.jpg)
