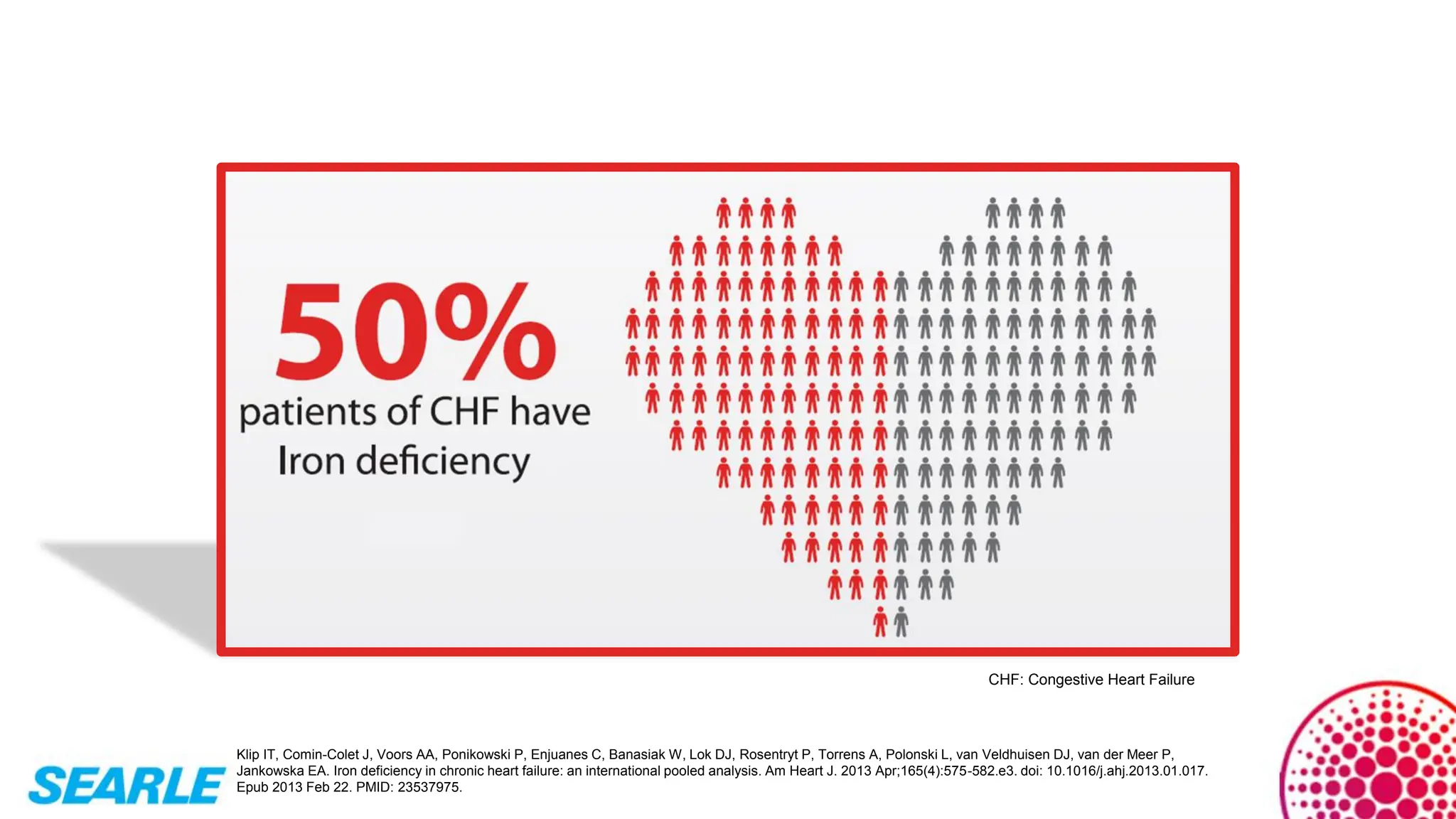
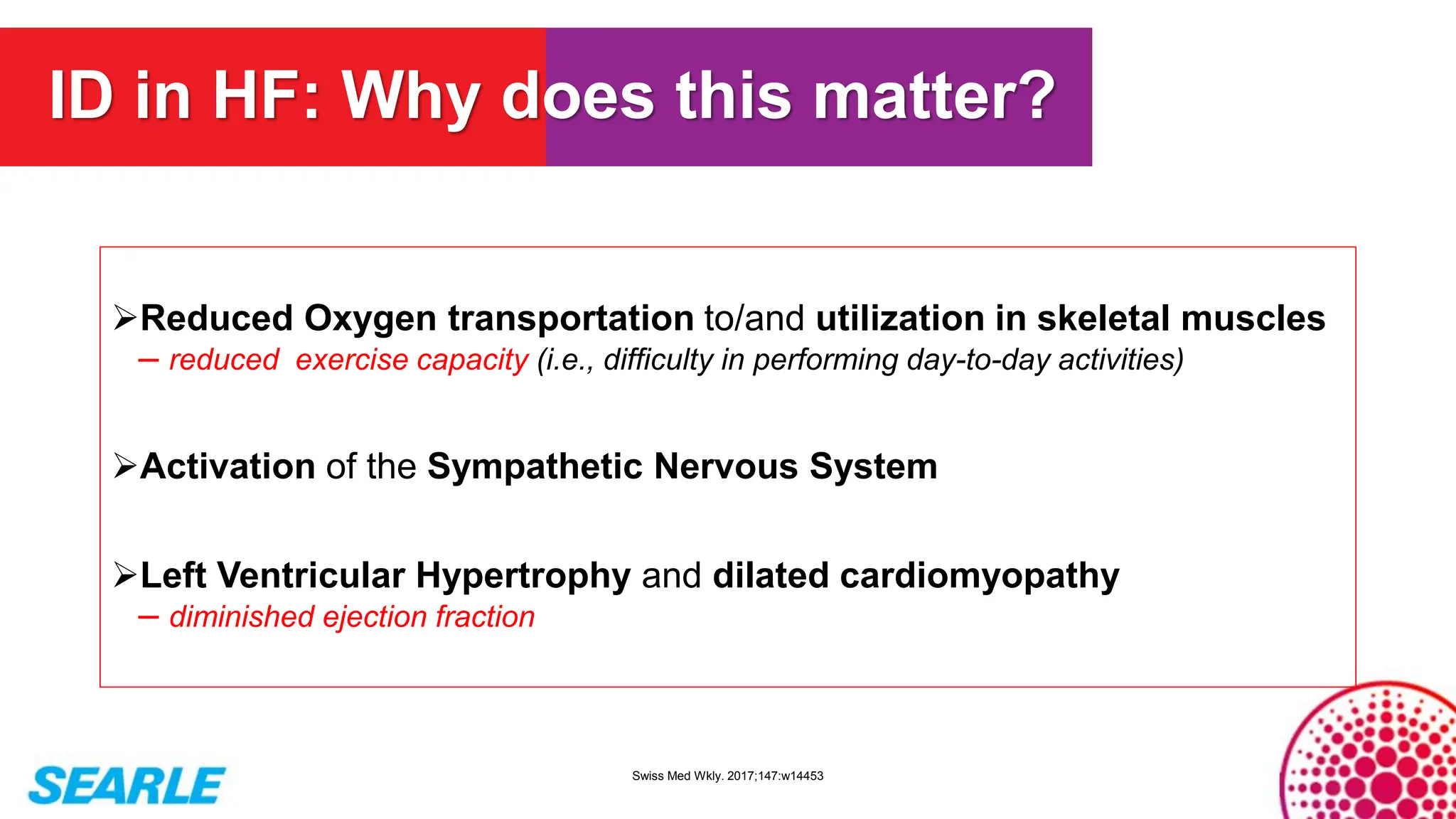
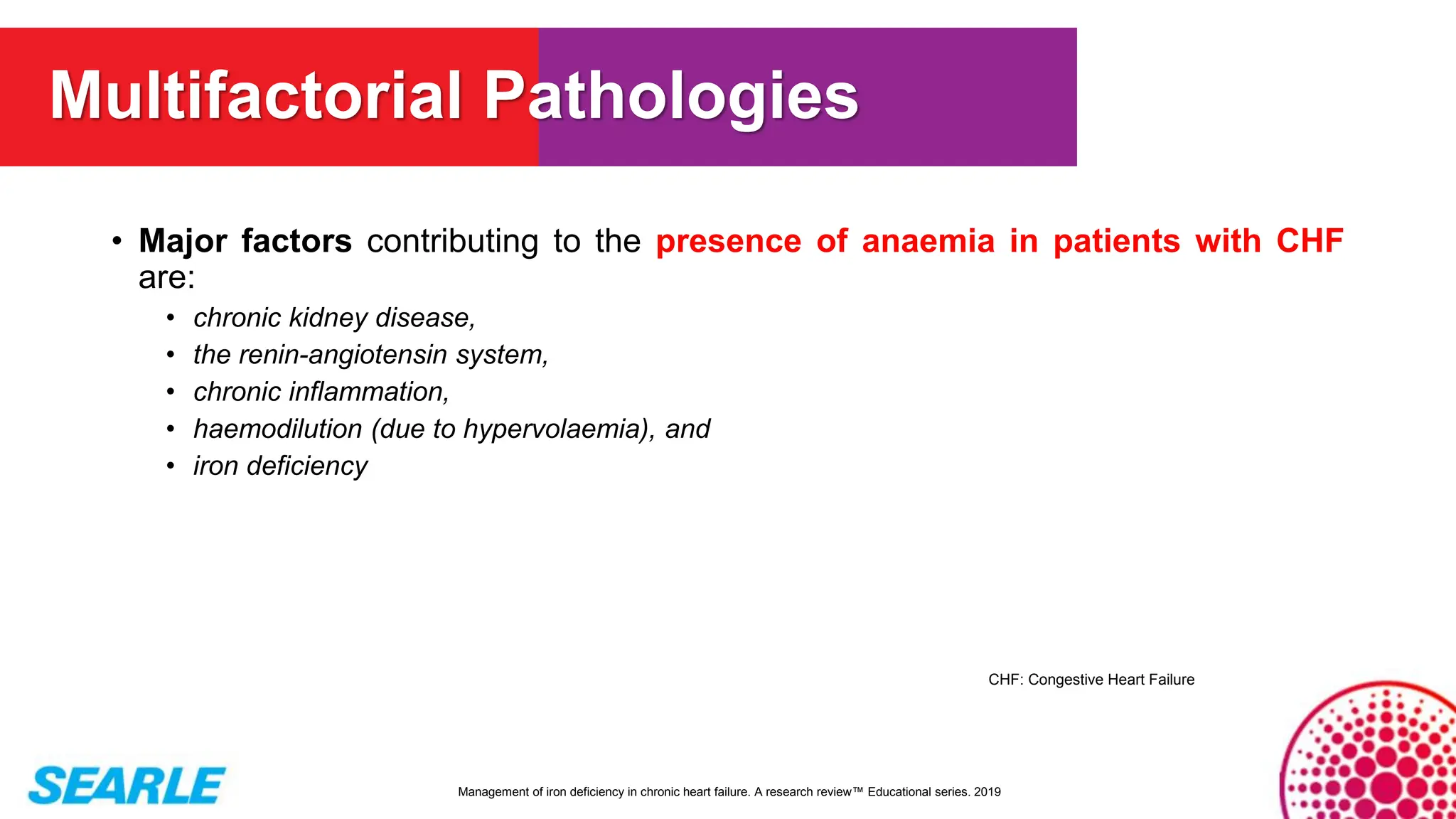
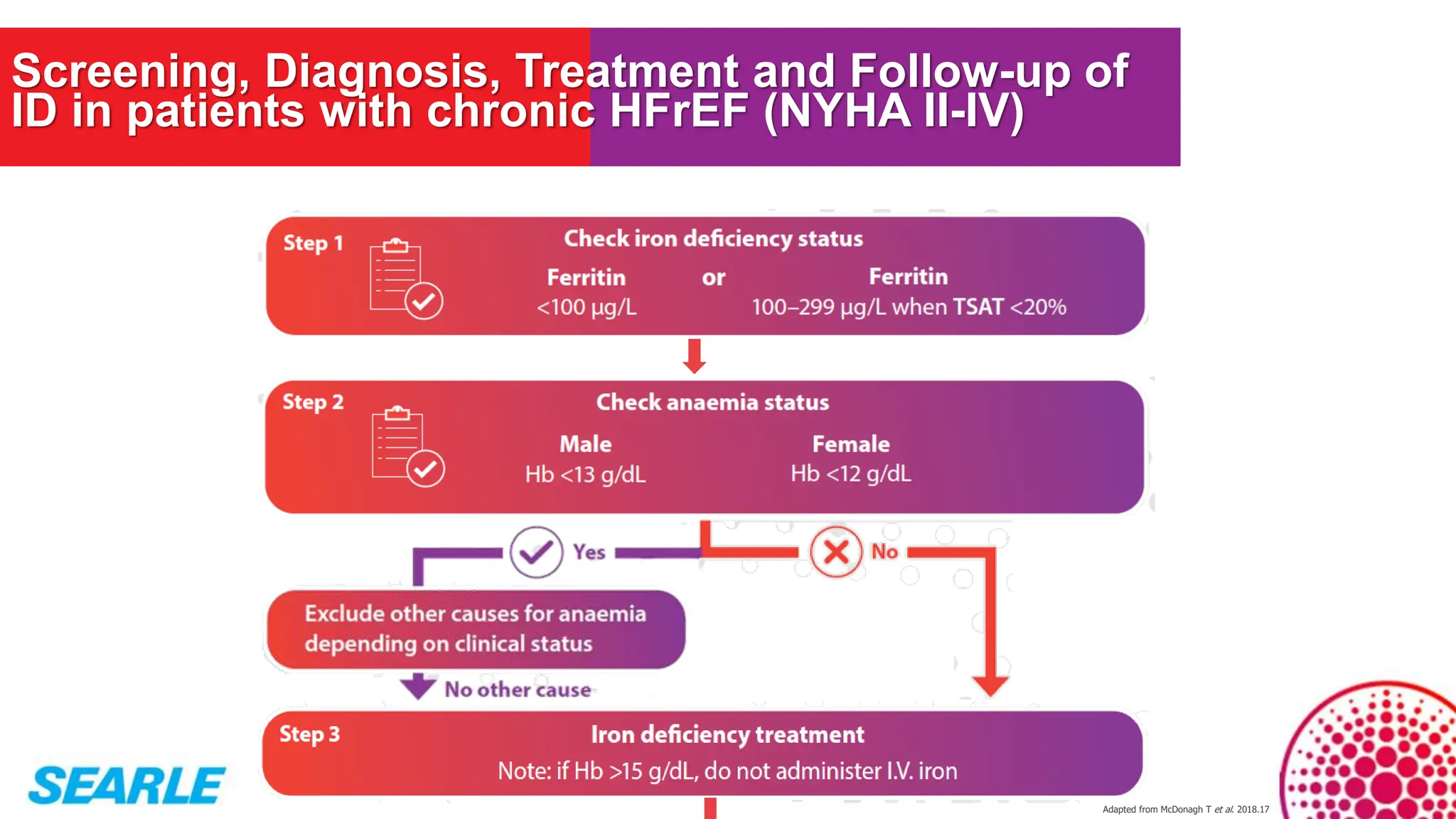
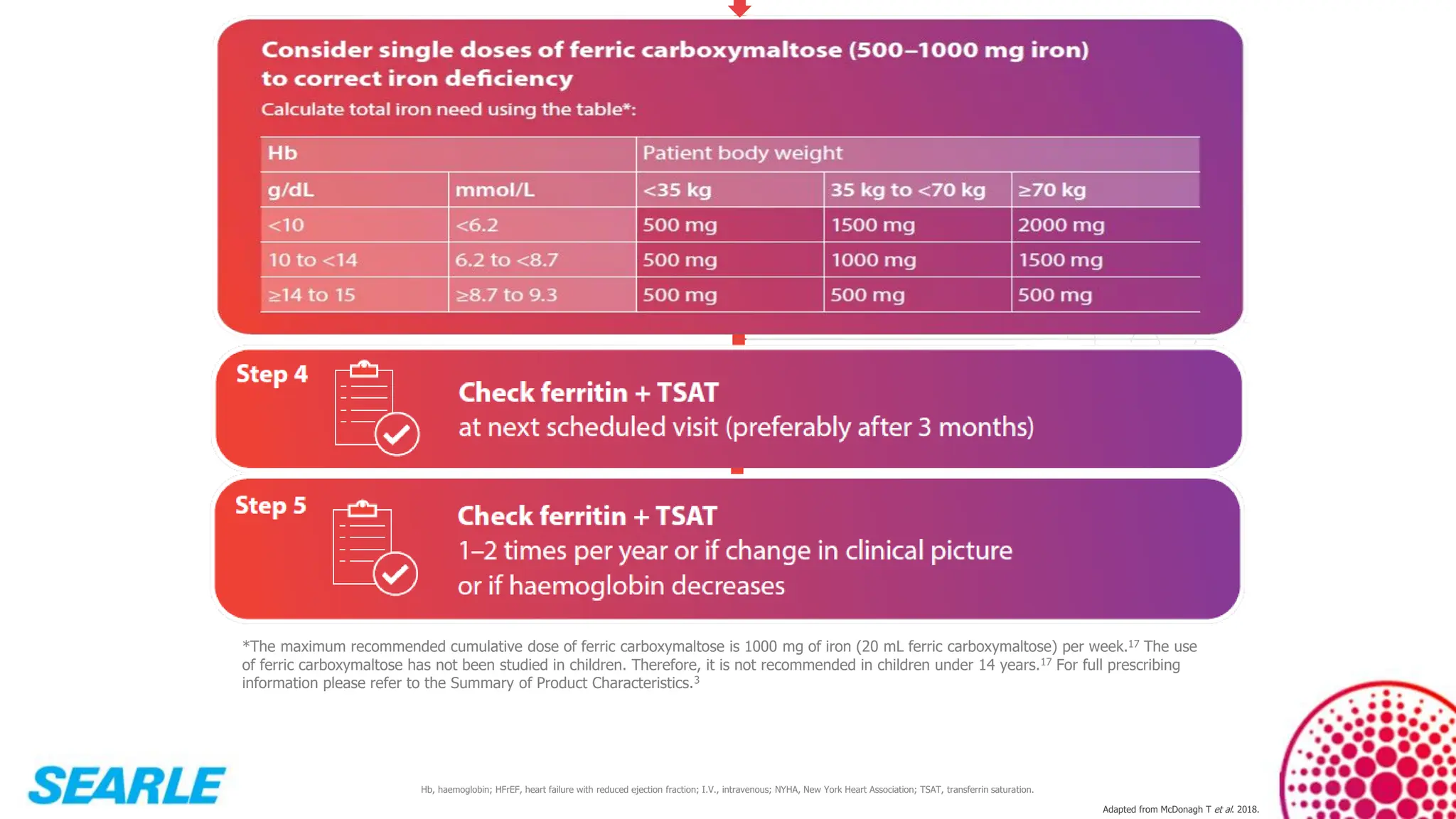
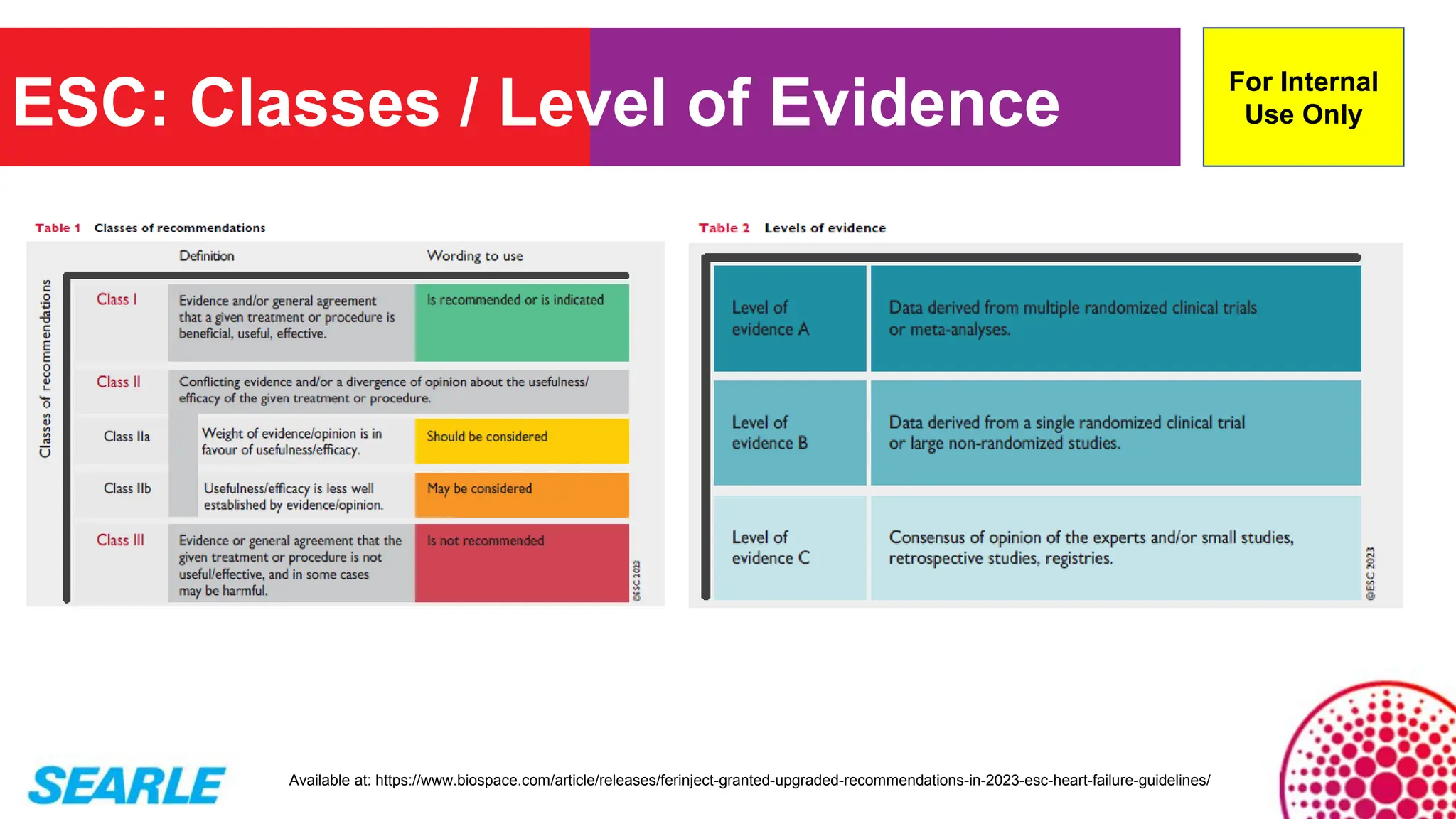
MiniTool Partition Wizard is a disk partition management software for Windows that allows users to create, resize, format, convert, and recover partitions without data loss. It supports HDDs, SSDs, USB drives, and RAID configurations, making it useful for both home users and IT professionals.
https://softwarepk.com/minitool-partition-wizard-crack/