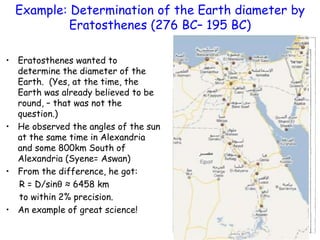
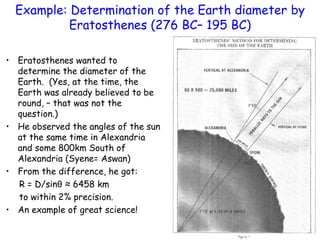
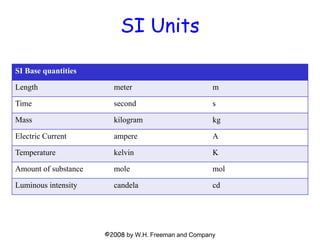
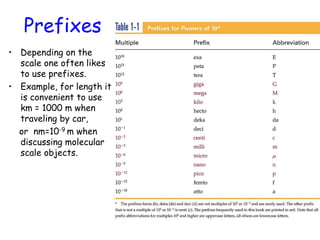
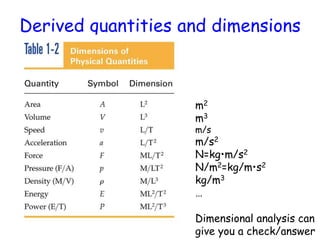
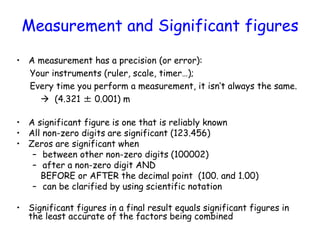
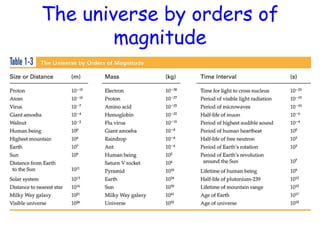
This document provides information about a Physics 201 course covering topics like kinematics, dynamics, statics, fluids, and oscillations. It discusses the textbook, homework assignments on WebAssign, labs, discussions, and teaching assistants. Physics is described as the basic science that includes concepts from mechanics, optics, electricity and magnetism, atomic and nuclear physics. Examples are given of how scientific theories are developed from observations and experiments, and how Eratosthenes calculated the diameter of the Earth in the 3rd century BC. The document also covers units in the SI system, prefixes, conversions between units, derived quantities, dimensions, and measurement with significant figures.