Embed presentation
Download to read offline

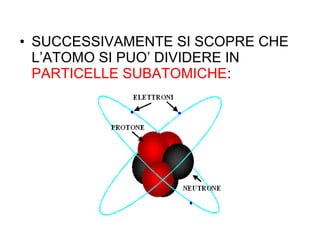



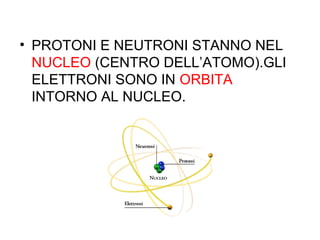




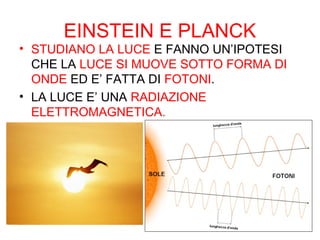

This document summarizes the history of discoveries about the structure of atoms. It explains that originally an atom was thought to be indivisible, but it was later discovered that atoms can be divided into subatomic particles. Protons have a positive charge, electrons have a negative charge, and neutrons have no charge. Protons and neutrons are located in the nucleus at the center of the atom, while electrons orbit around the nucleus. Scientists like J.J. Thomson, Rutherford, Einstein, Planck, and Bohr further expanded the understanding of atomic structure through experiments and theories about electrons, light, and energy levels within atoms.













