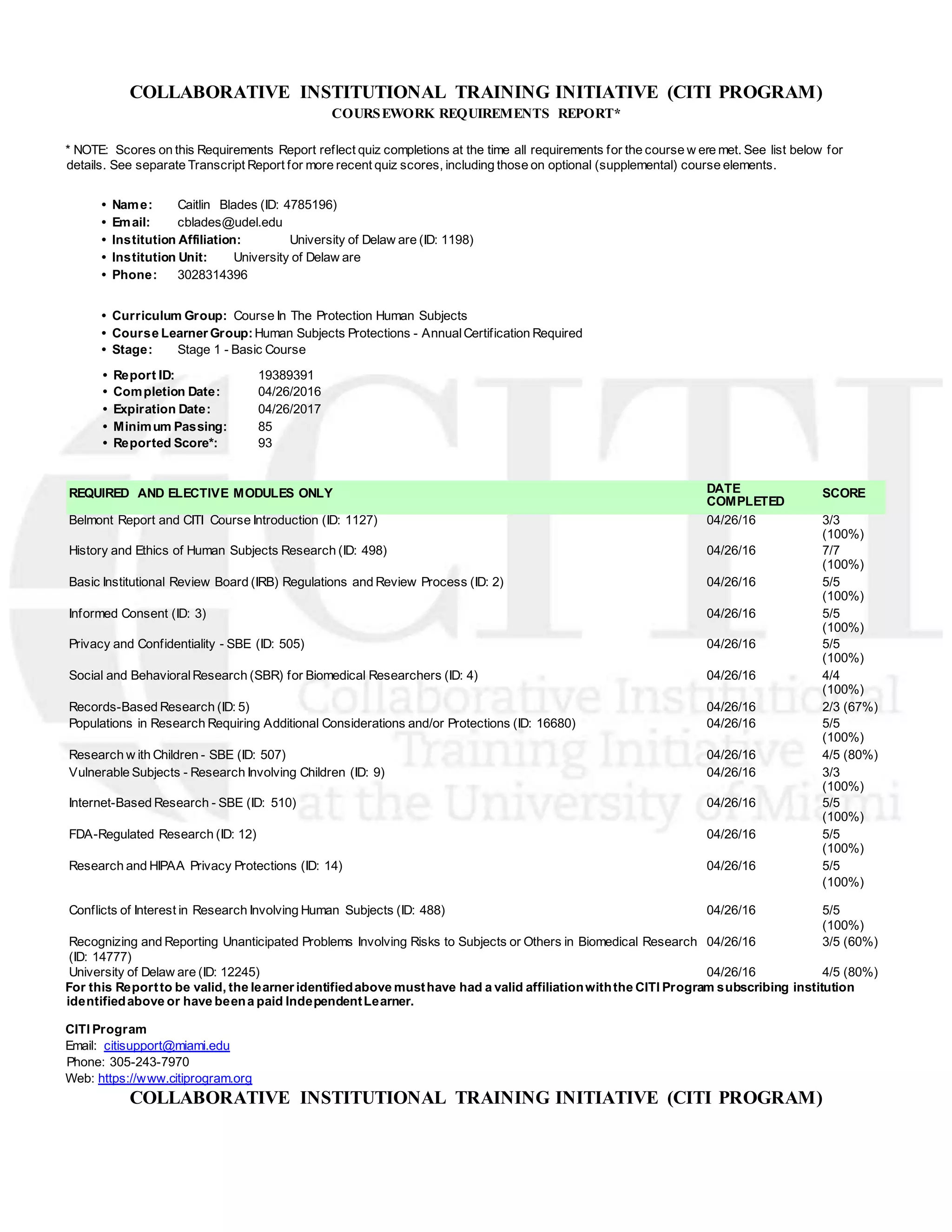
This document is a report from the Collaborative Institutional Training Initiative (CITI) program documenting the coursework completed by Caitlin Blades on human subject protections. It shows that she completed 16 required and elective modules with scores ranging from 60% to 100% and an overall score of 93%. A transcript report also documents additional more recent quiz scores, including on supplemental modules, with an overall score of 91%. Both reports list the module names and dates and scores for each item completed by Caitlin Blades to satisfy her training requirements for research involving human subjects.