Embed presentation
Download to read offline


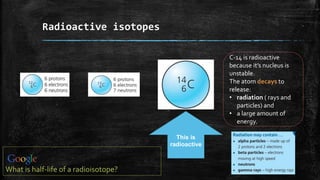


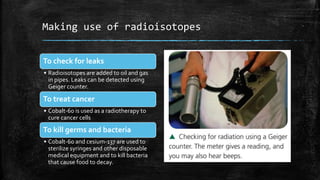





Isotopes are atoms of the same element that have different numbers of neutrons. Radioactive isotopes such as carbon-14 are unstable, causing their nuclei to decay and release radiation and energy. Radioisotopes have a measurable half-life and can be used to detect leaks in pipes, treat cancer through radiotherapy, and sterilize medical equipment by killing germs and bacteria.










