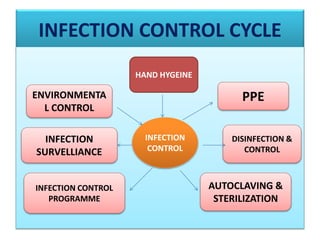
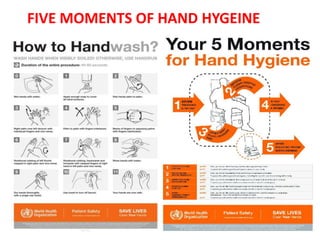
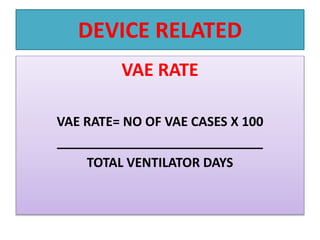
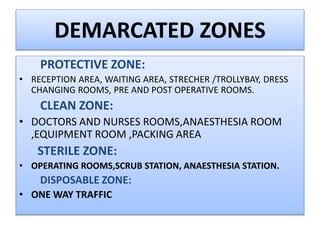
This document provides a summary of infection prevention and control practices at a healthcare facility. It discusses proper hand hygiene techniques, appropriate use of personal protective equipment, cleaning and disinfection protocols, sterilization procedures, healthcare-associated infection surveillance including rates of SSIs and device-related infections, environmental cleaning, biomedical waste management, and needlestick safety. The goal is to outline an infection control program and processes to prevent transmission of infections.