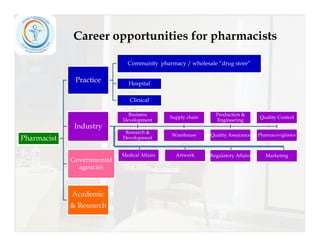
The document provides tips for improving CVs and developing careers in the pharmaceutical field. It recommends including personal details, education, computer skills, training courses, and checking for spelling errors on CVs. It also lists various career opportunities for pharmacists and free online trainings from global organizations covering medicine, pharmaceutical industry topics, and good manufacturing practices. Contact details are provided for networking and sharing beneficial resources.