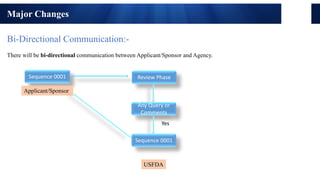
The document discusses the key changes and objectives of ICH eCTD 4.0. Major changes include bi-directional communication between applicants and agencies, assigning each document a unique UUID number, consolidating multiple files into a single XML file, replacing the operation attribute with options for replacing or appending documents, and allowing metadata to be edited after submission. The objectives are to improve processing and review between applicants and agencies and make submissions and publishing more effective. Adoption of ICH eCTD 4.0 will require system upgrades and additional archival space.