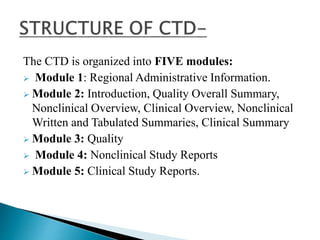
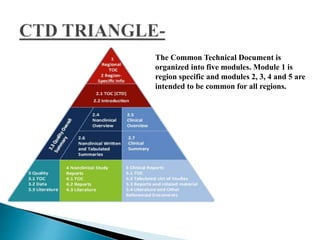
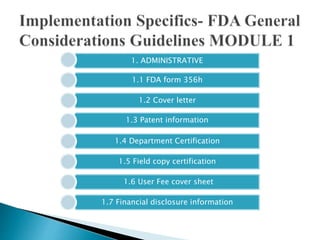
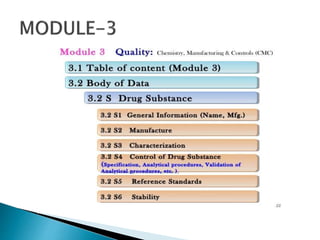
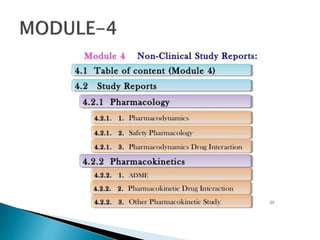
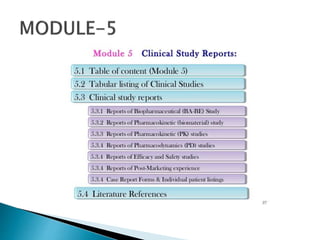
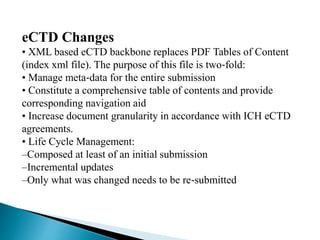
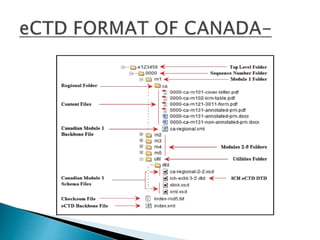
The document outlines the Common Technical Document (CTD) and its electronic counterpart, the Electronic Common Technical Document (eCTD), emphasizing their roles in harmonizing pharmaceutical registration processes internationally. It details the structure of the CTD, which consists of five modules covering administrative information, summaries, quality assessments, and study reports, enabling efficient submission and review of medicines. The eCTD aims to enhance submission accuracy and decrease costs while facilitating better communication and information management in regulatory processes.