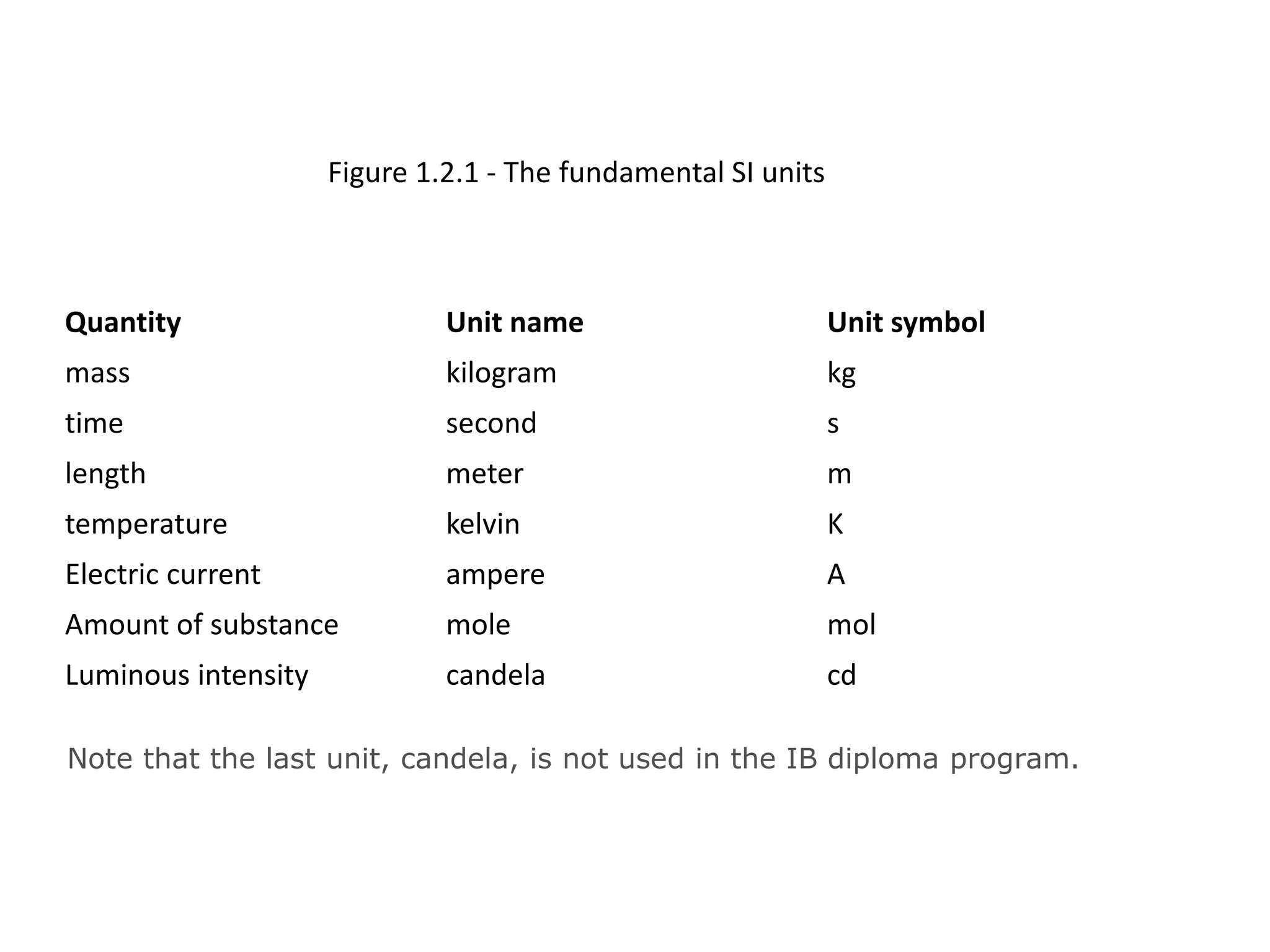
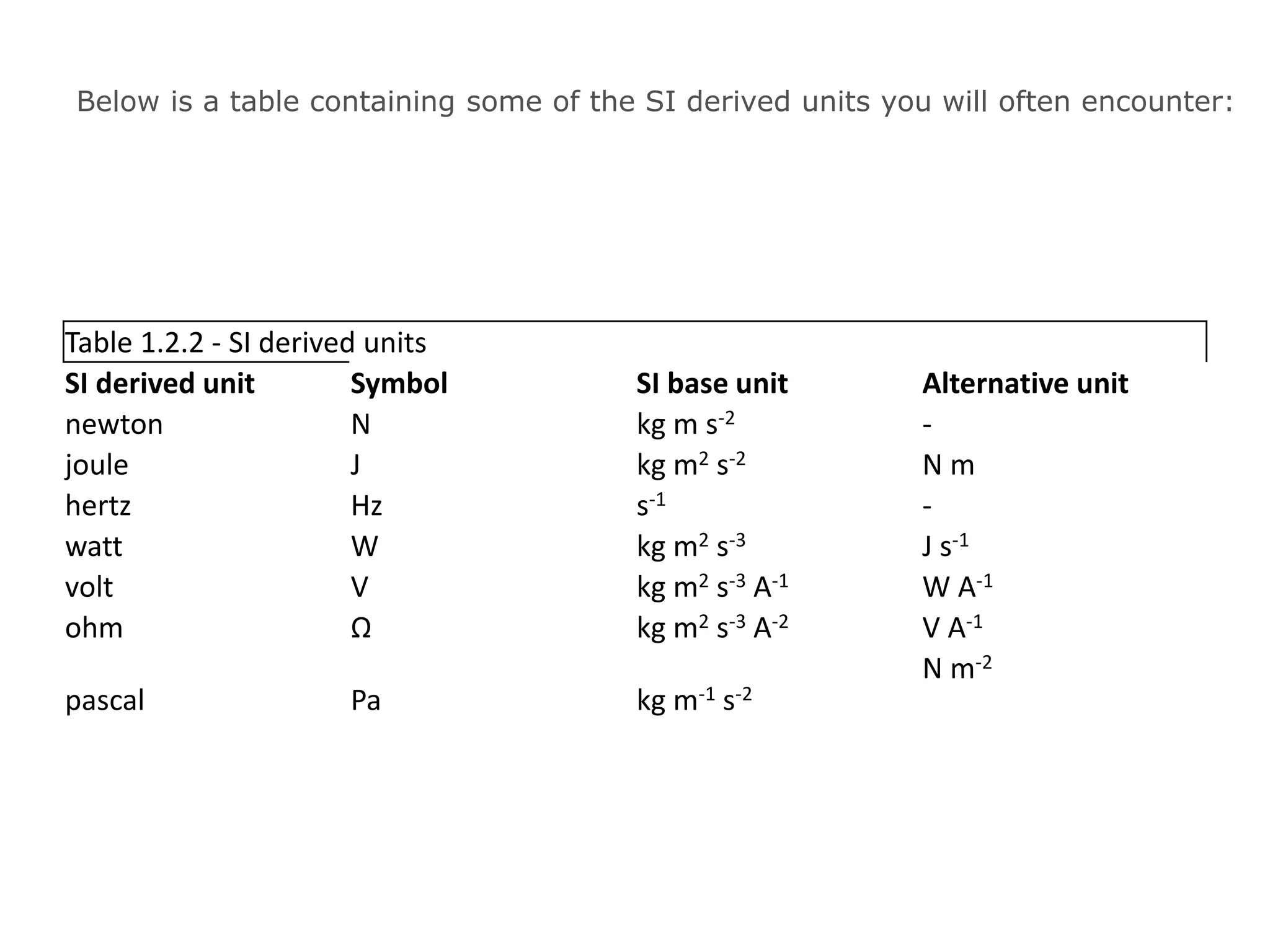
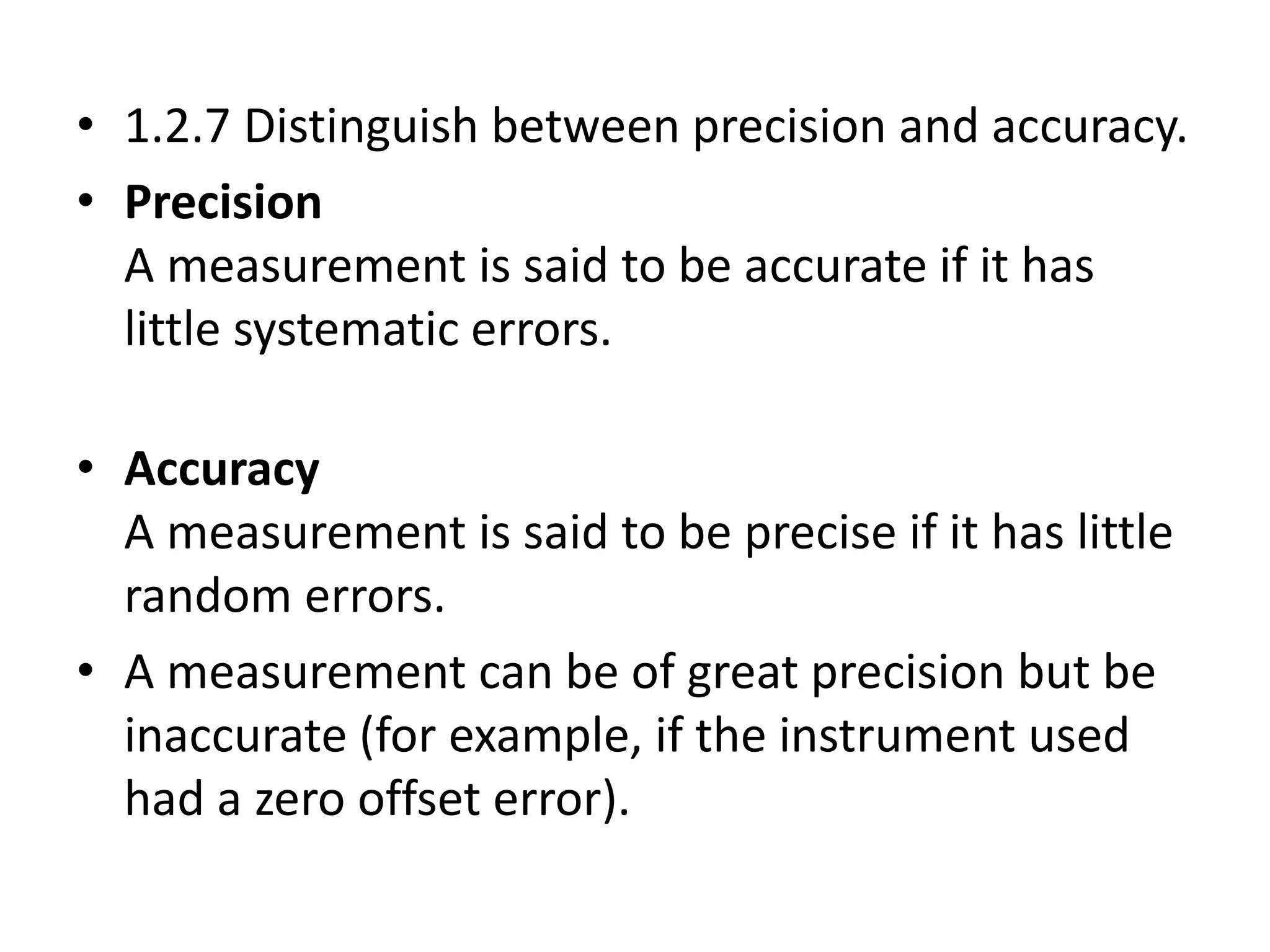
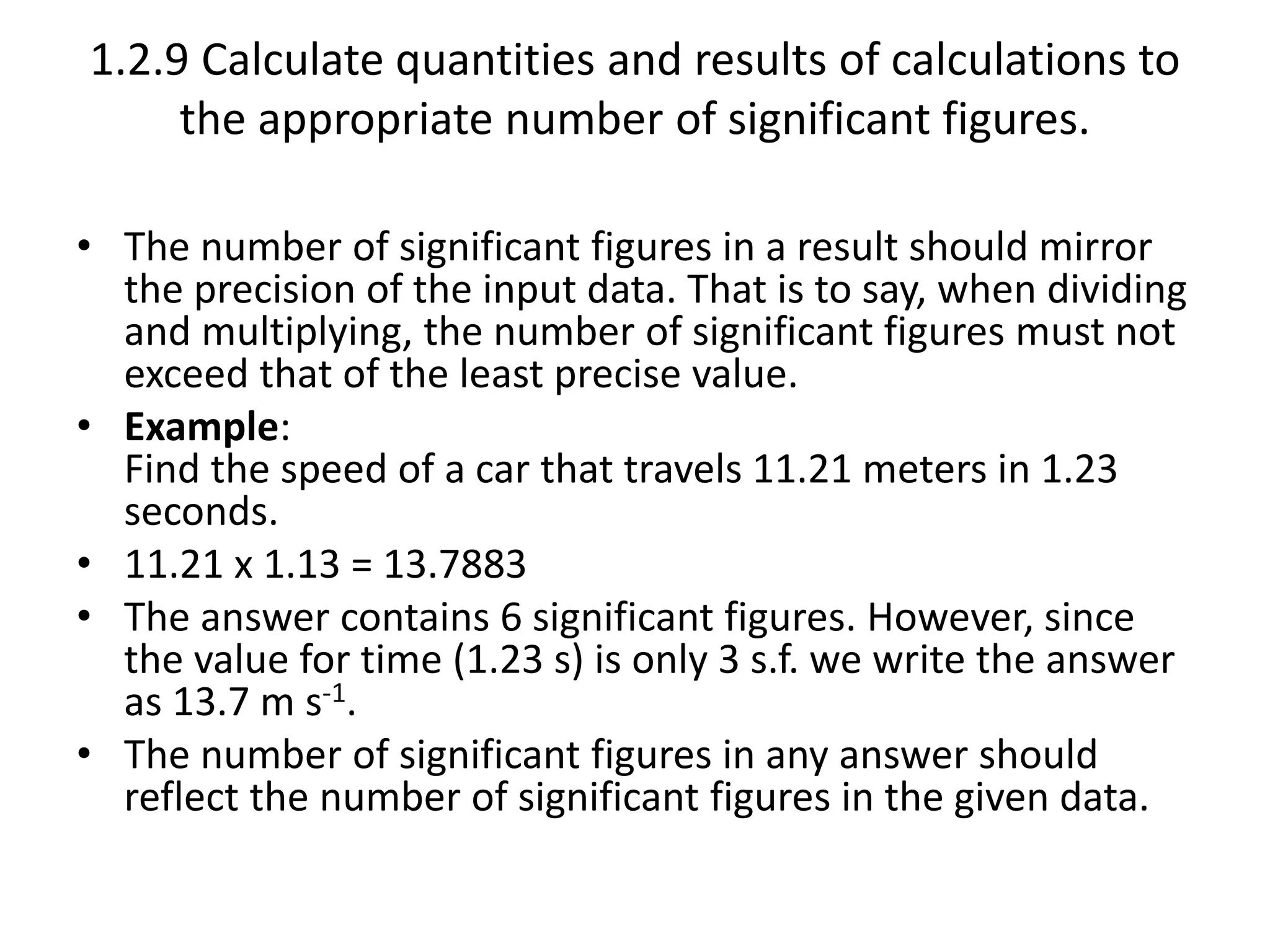
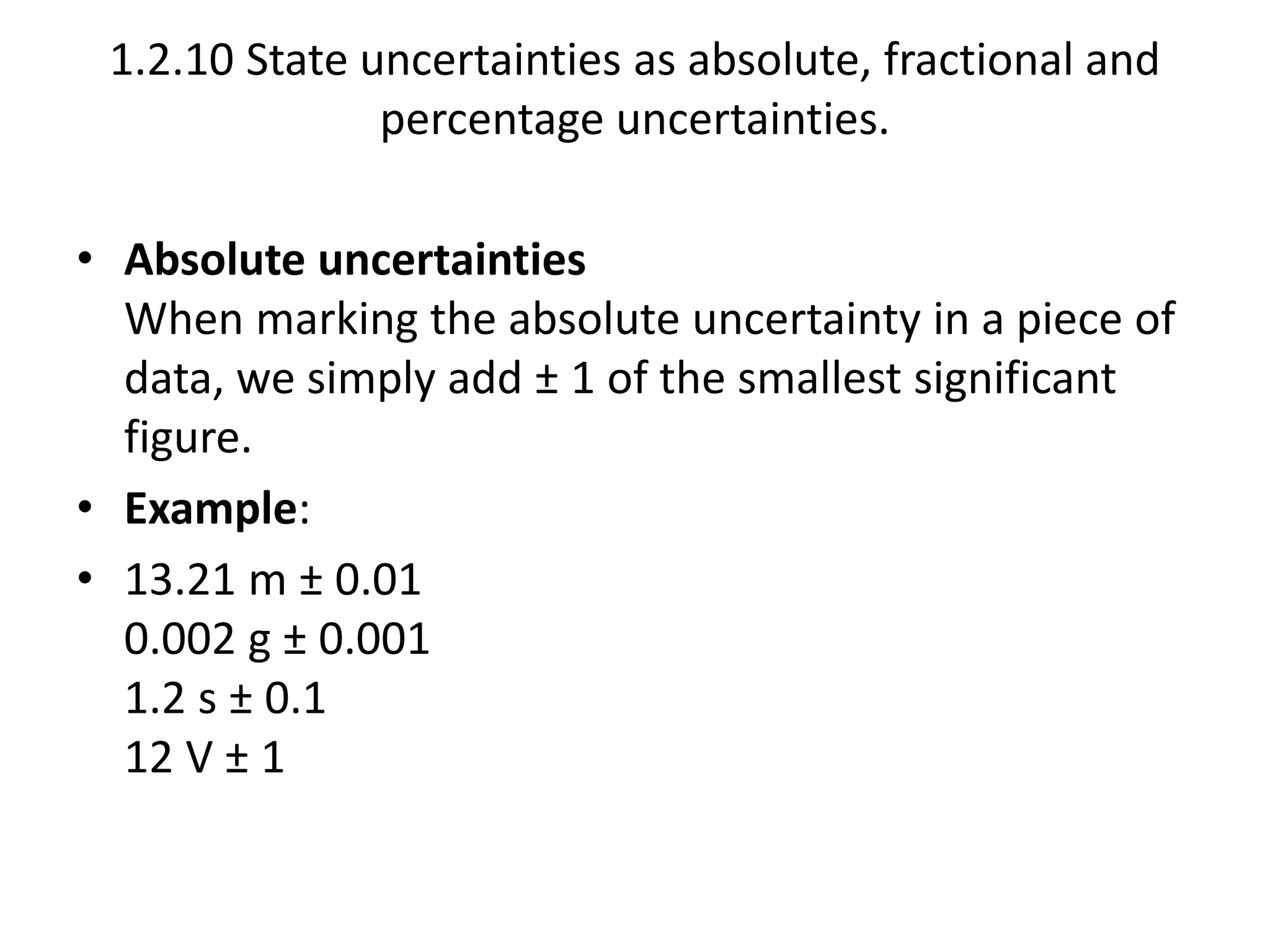
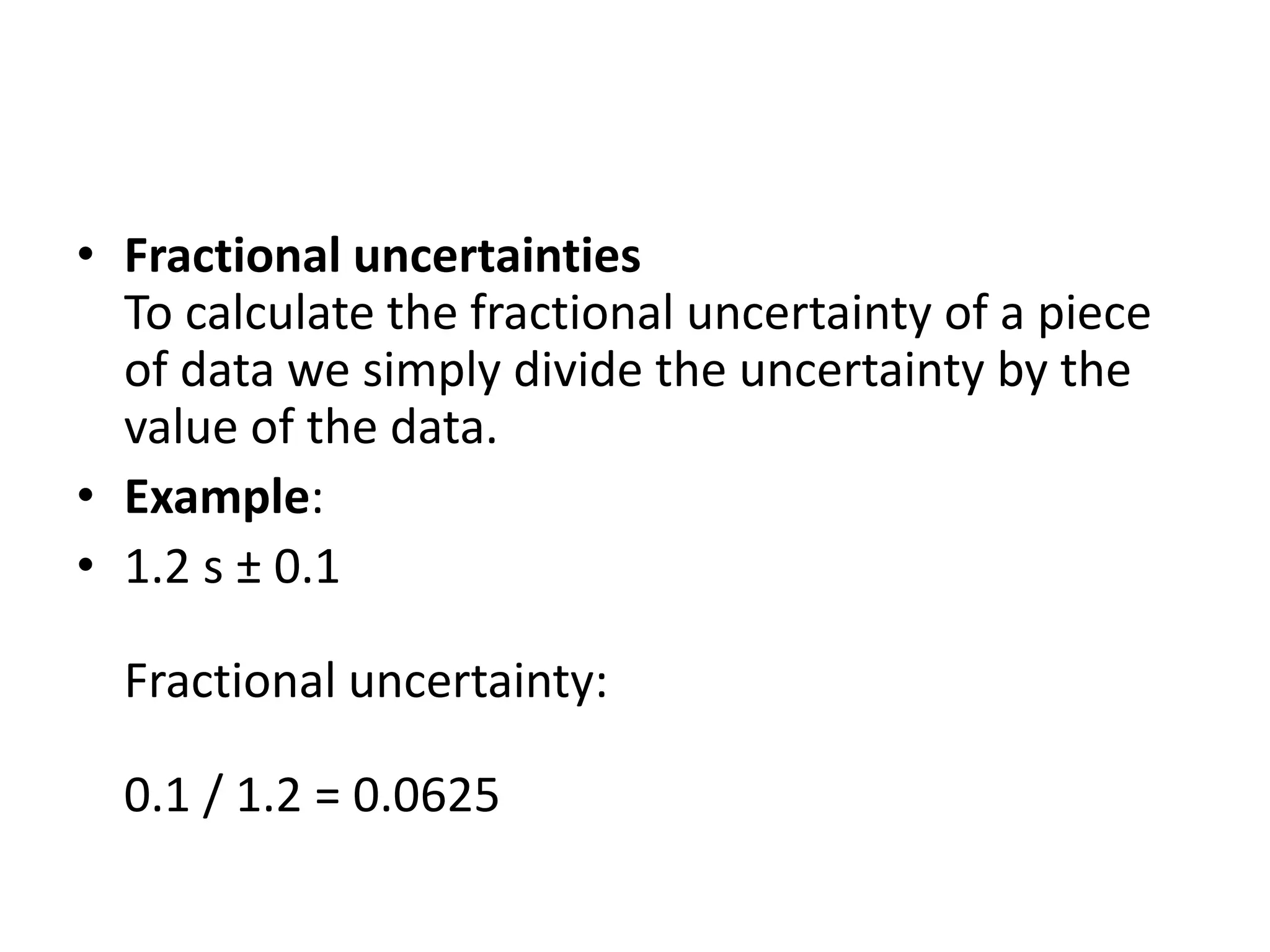
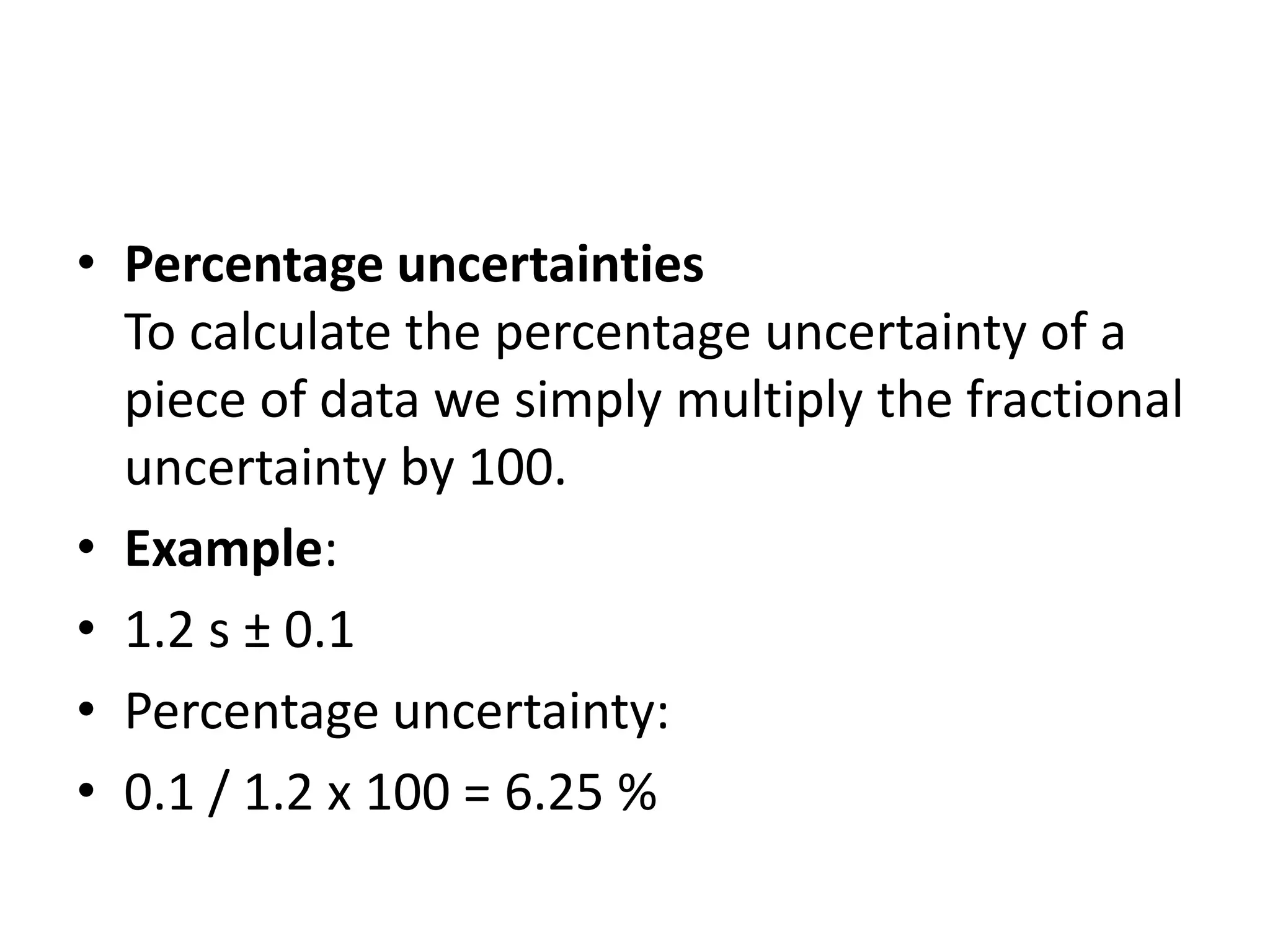
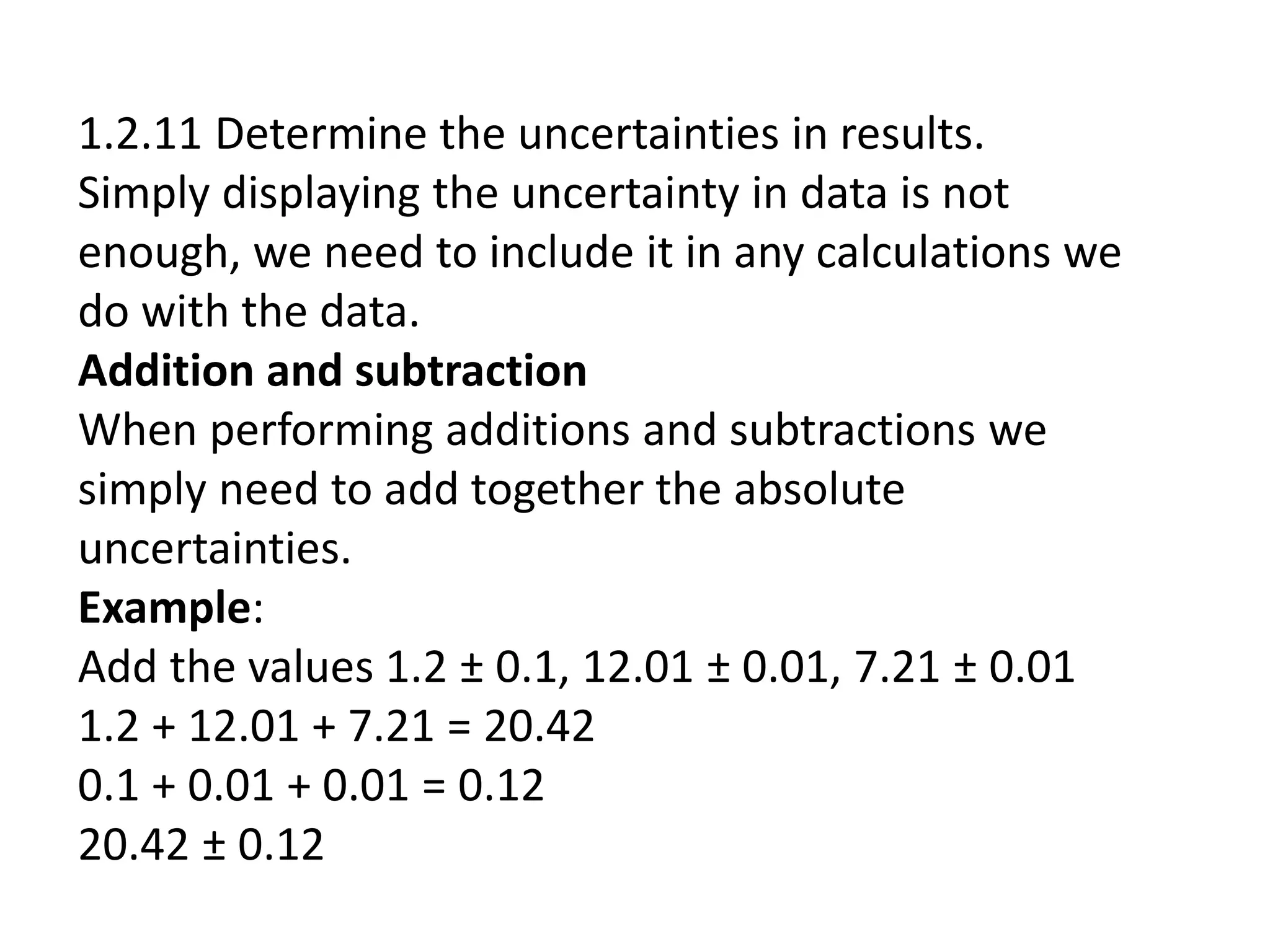
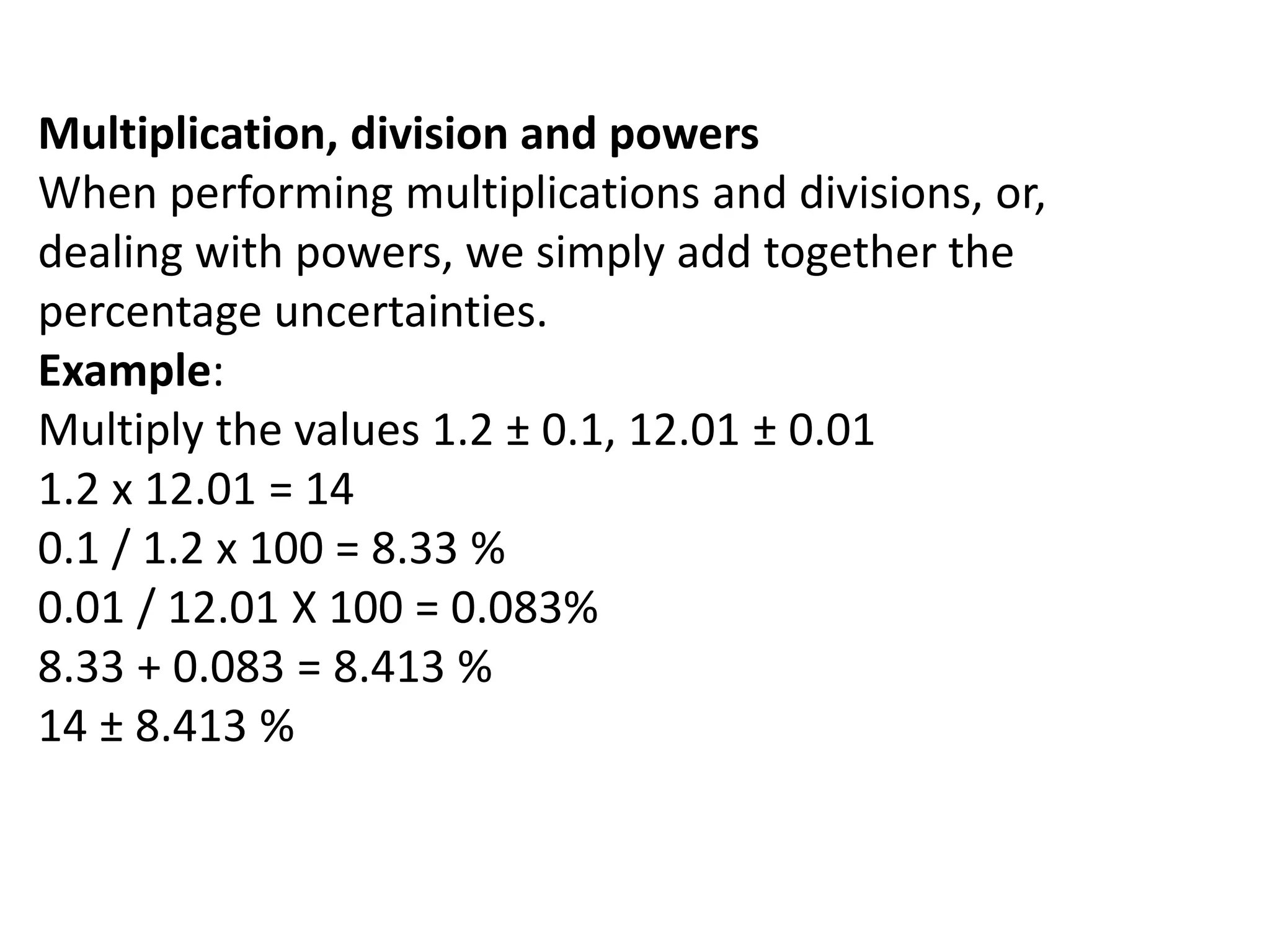
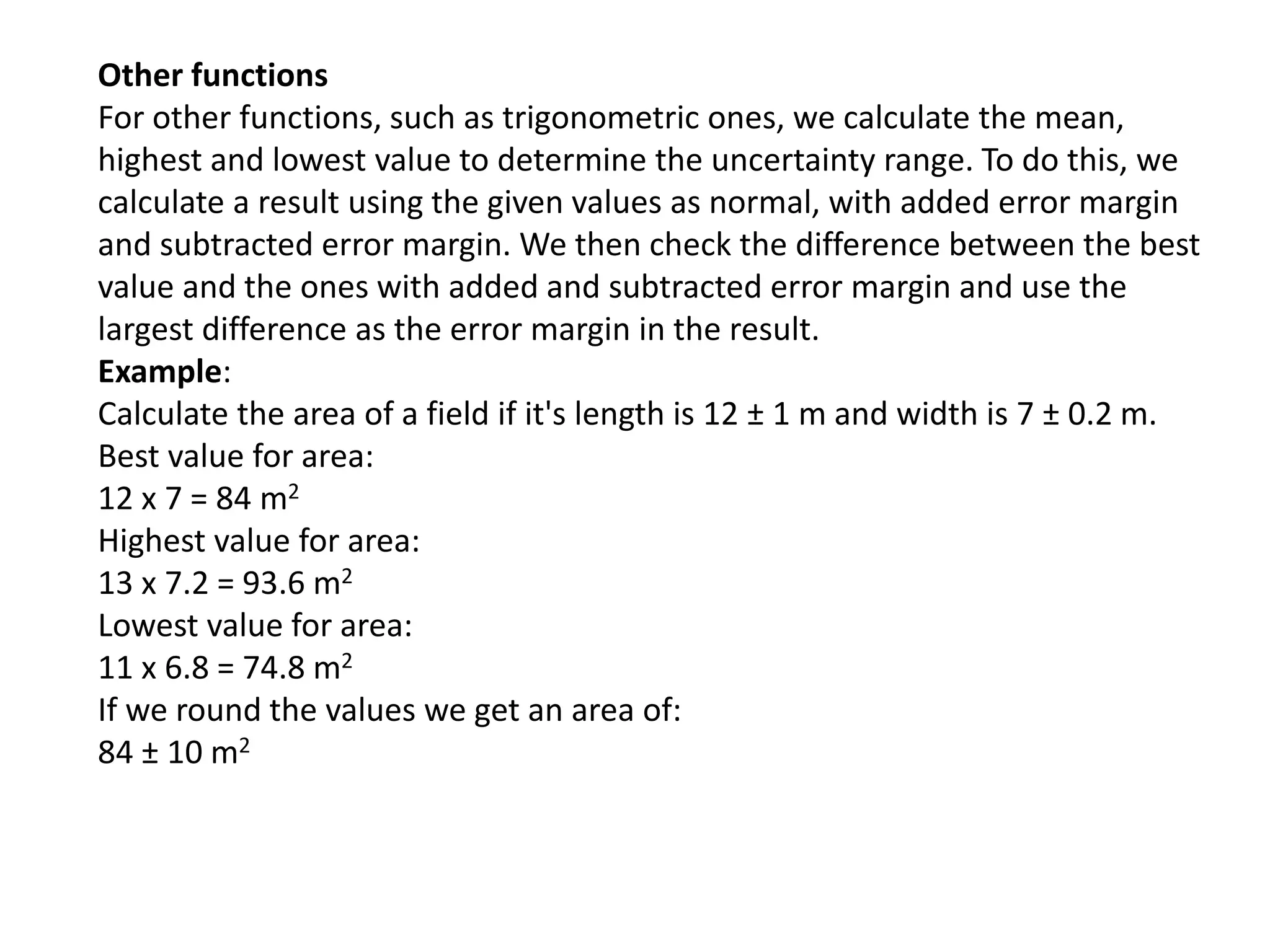
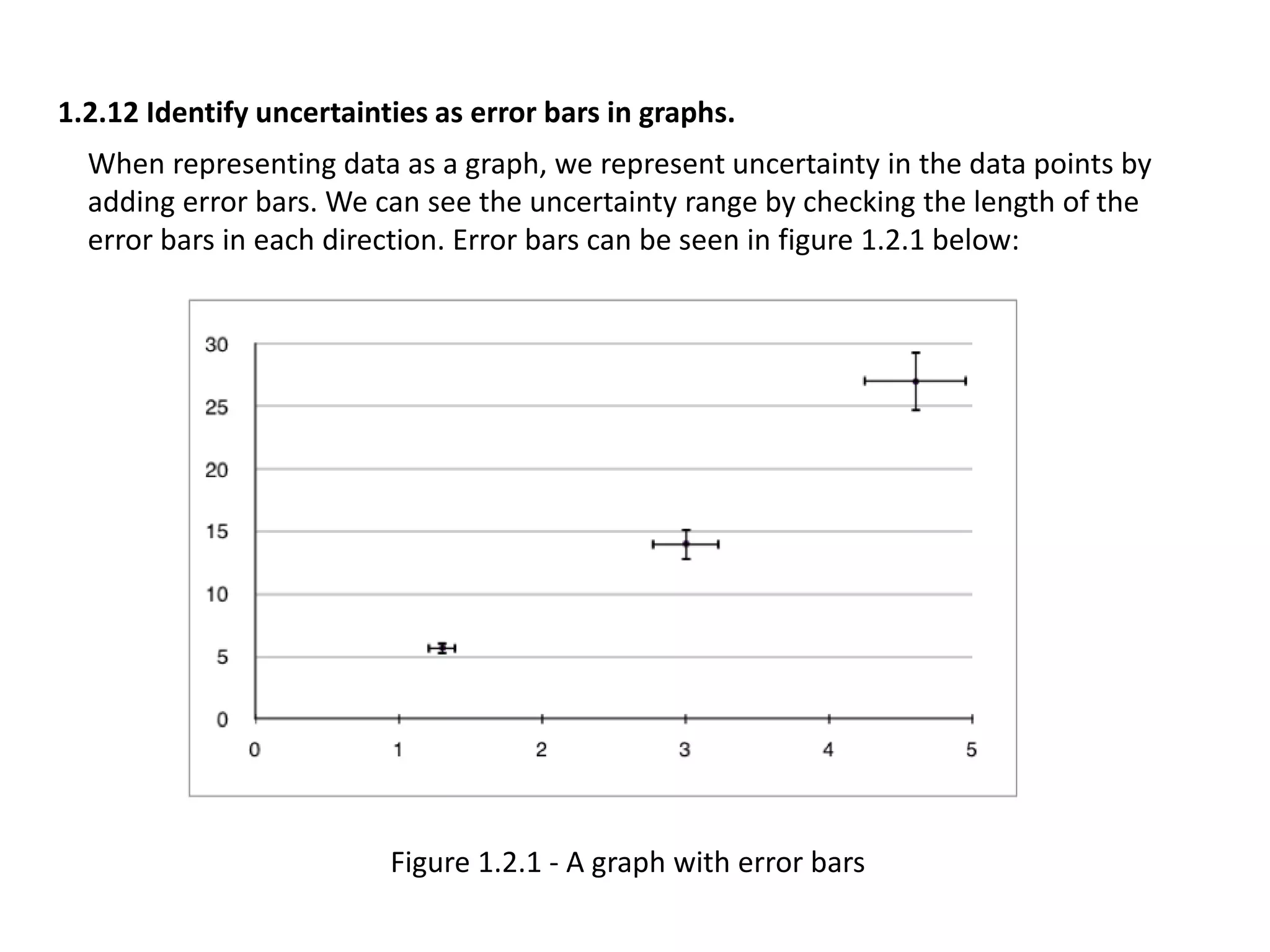
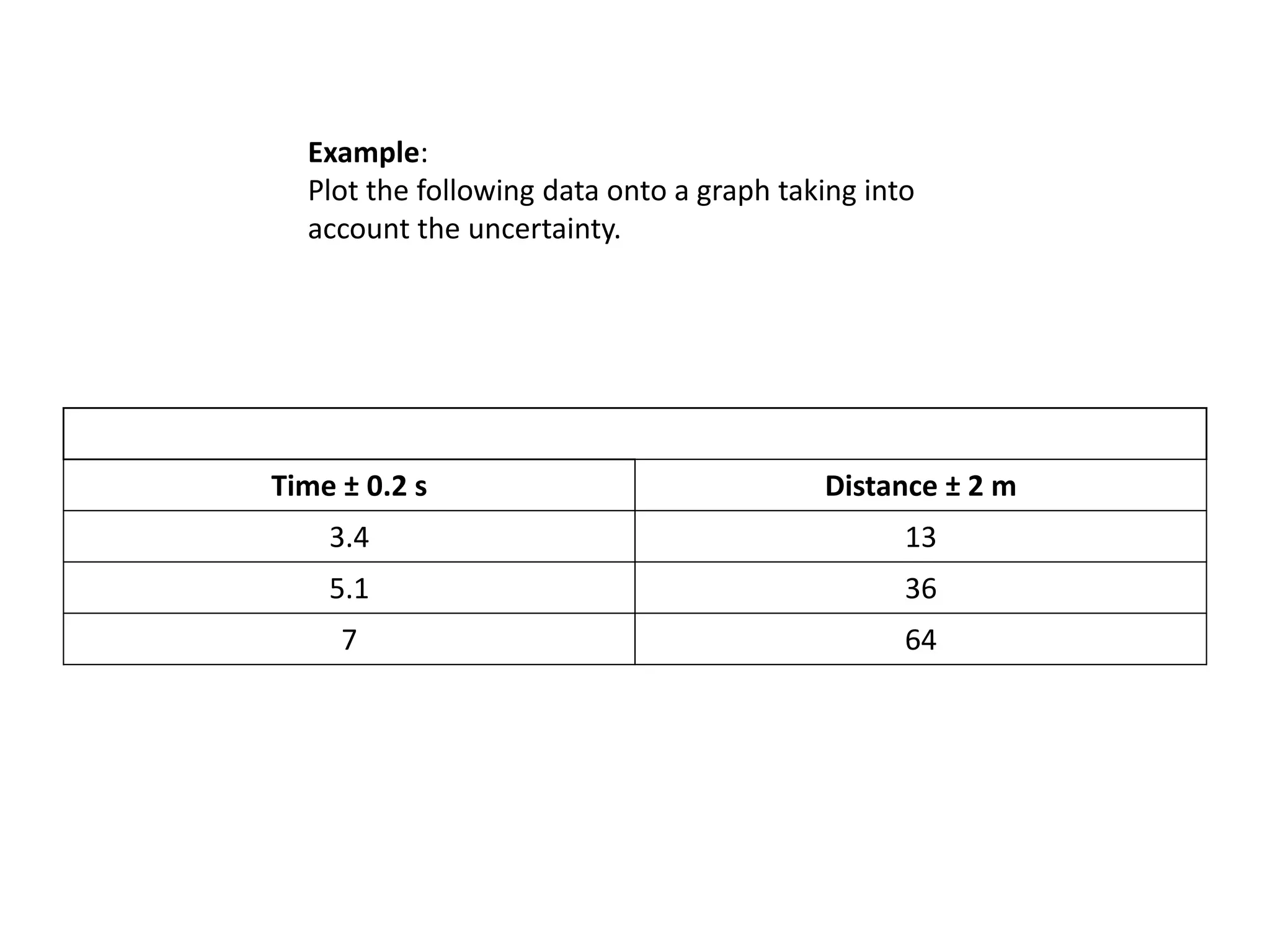
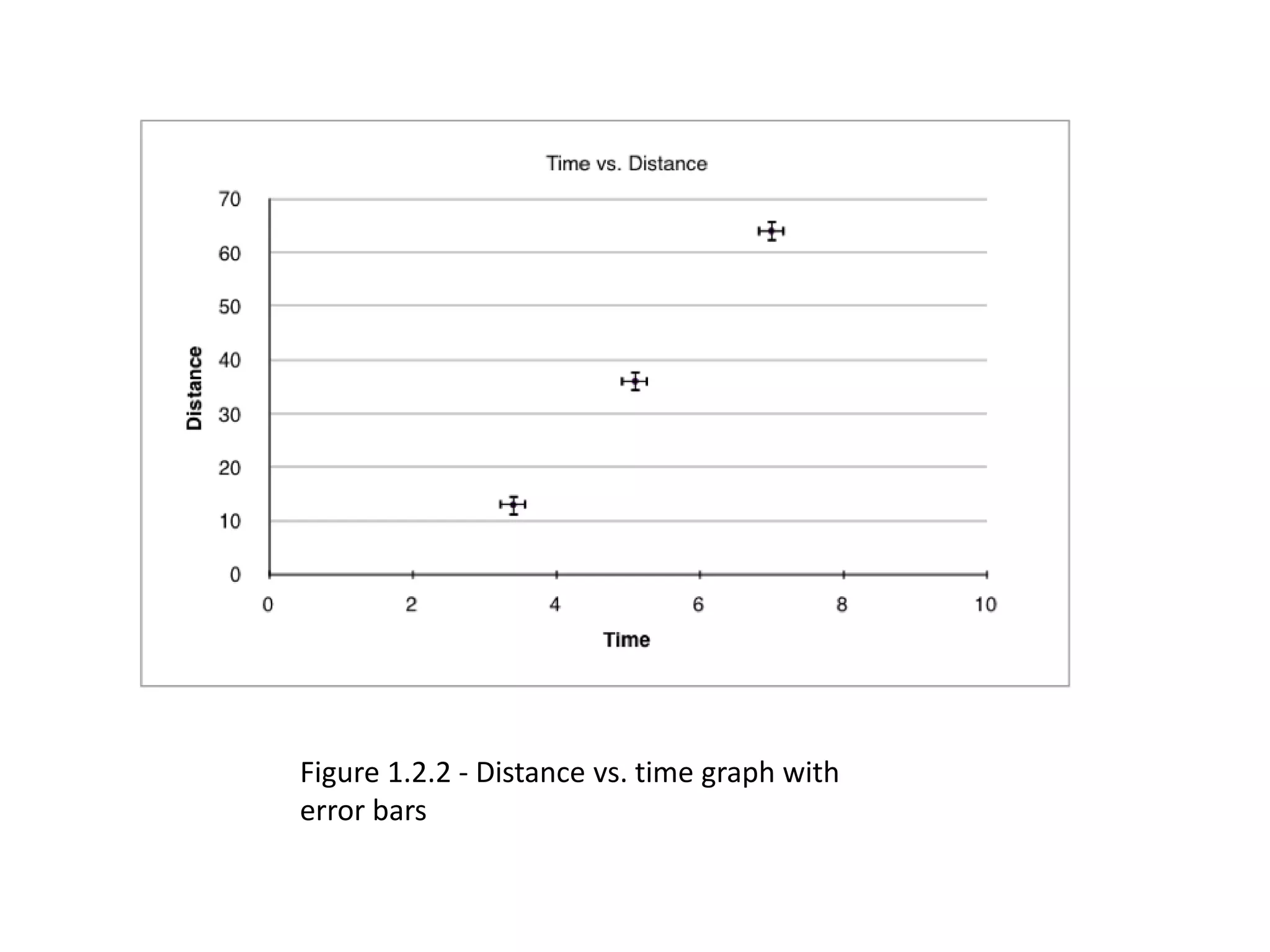
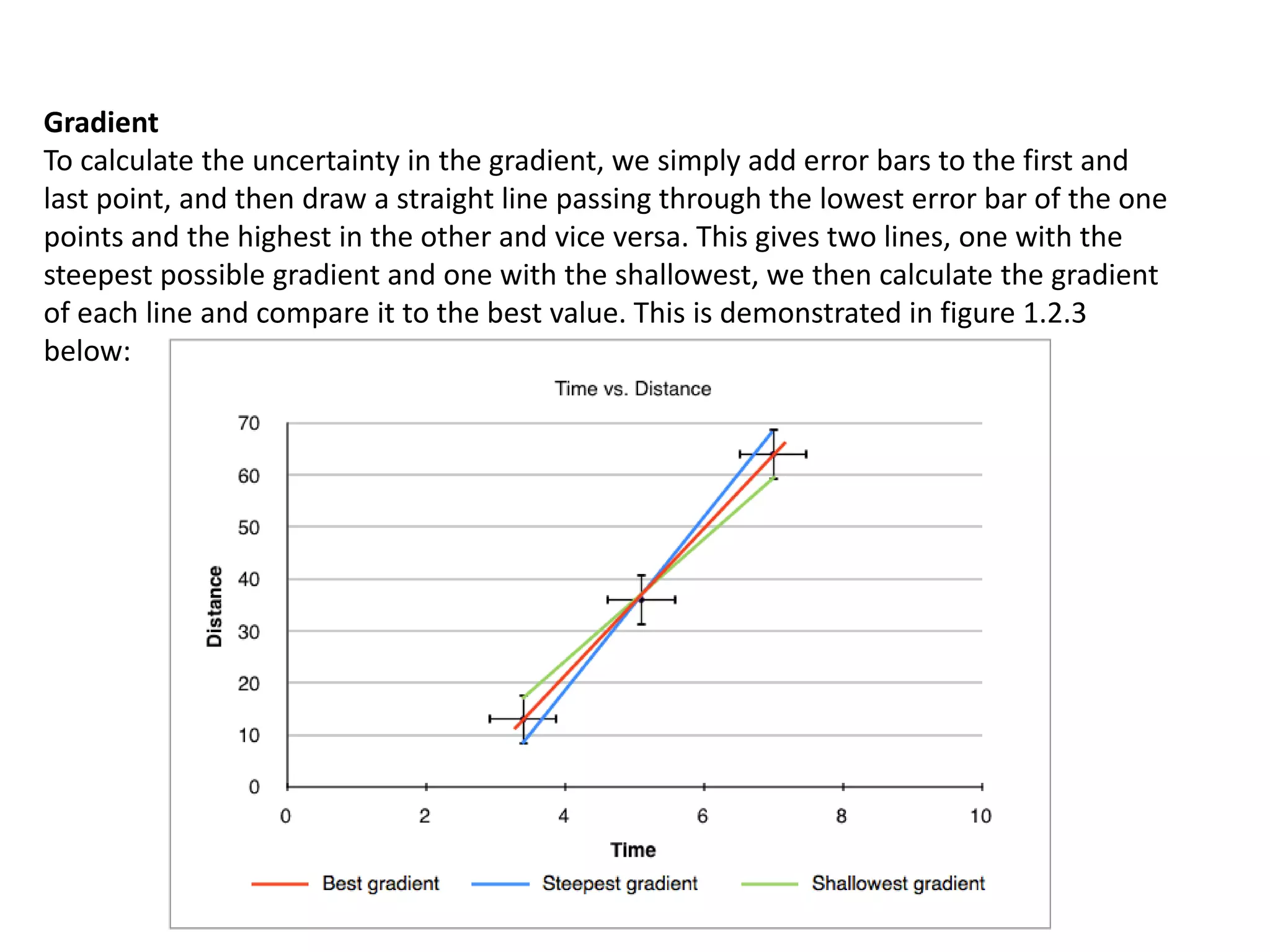
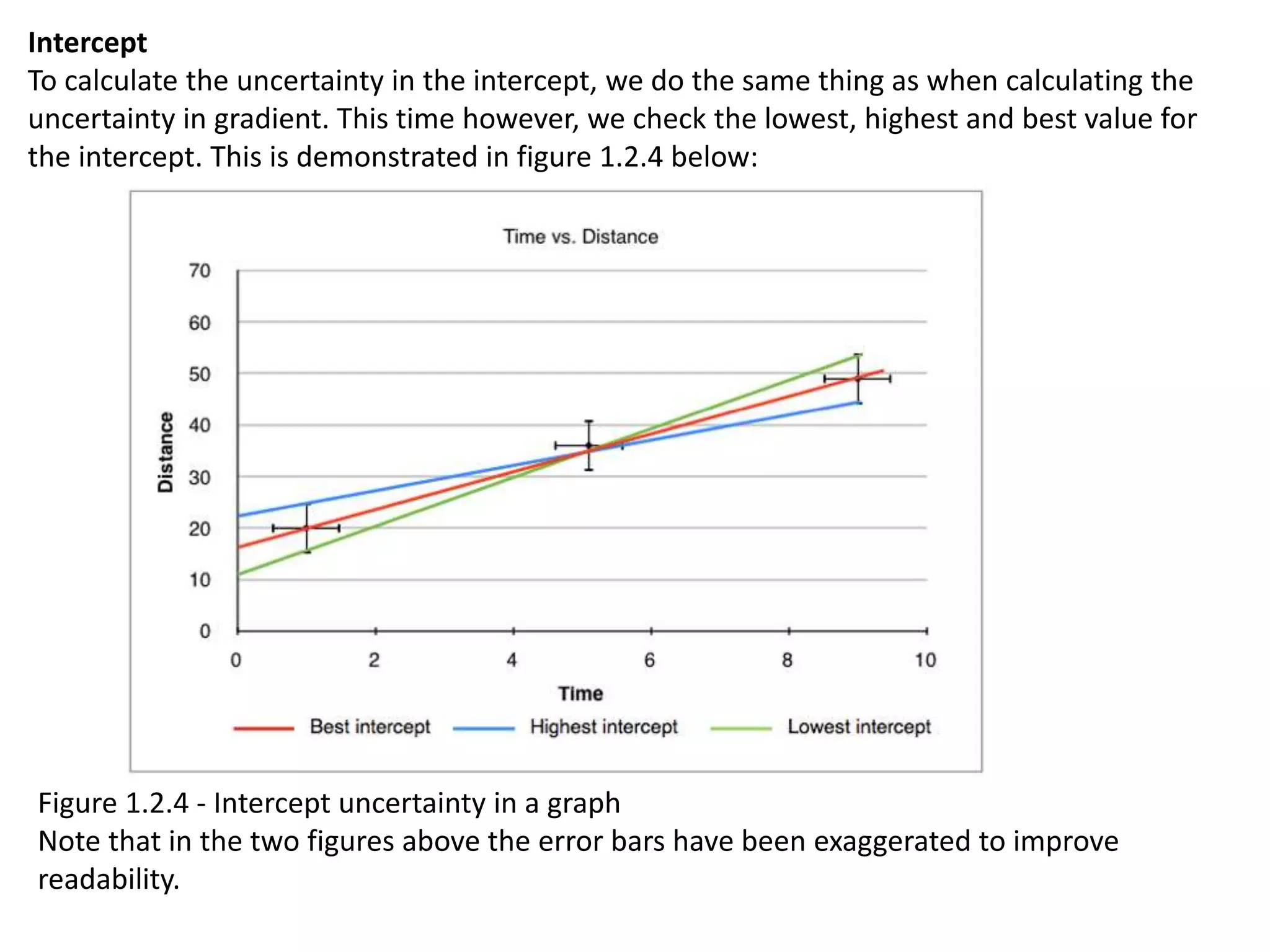
This document discusses measurement and uncertainties in physics. It covers:
1) Expressing quantities using orders of magnitude to easily understand scale.
2) The ranges of distances, masses, and times that occur in the universe from smallest to greatest.
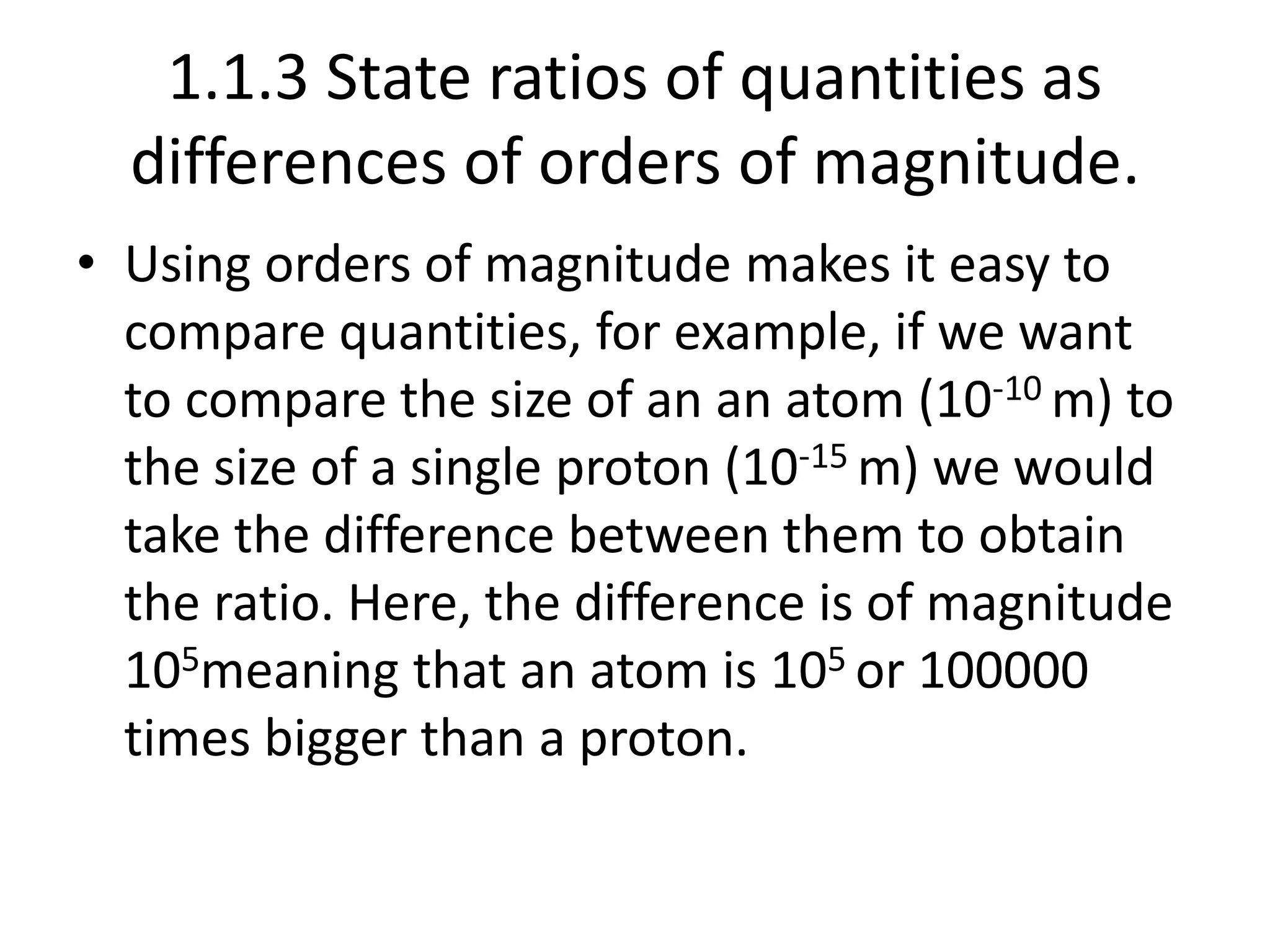
3) Calculating ratios as differences in orders of magnitude to compare quantities.