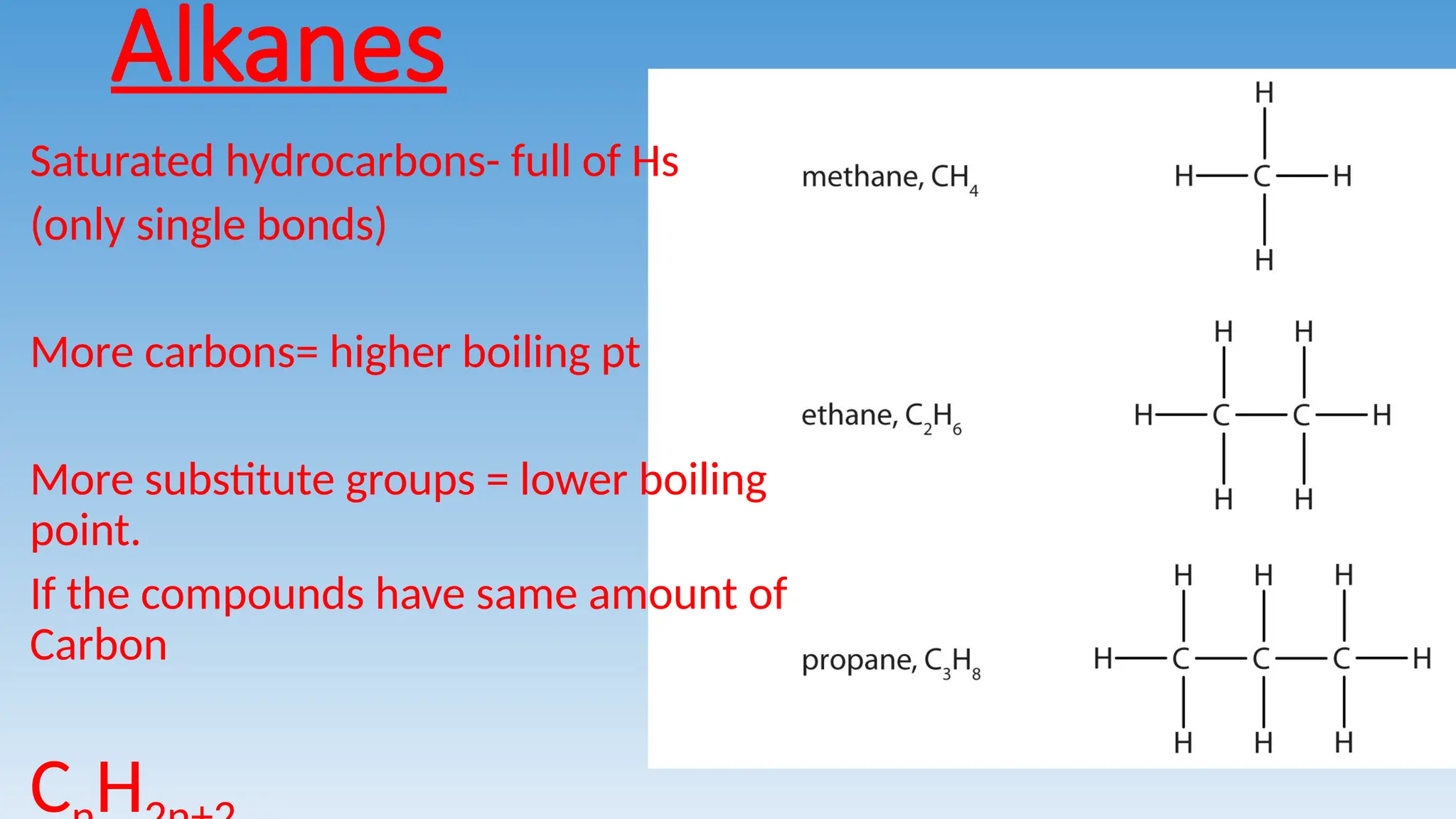
The document provides an overview of hydrocarbons, specifically focusing on alkanes, alkenes, and alkynes, explaining their formulas, bonding types, and naming conventions. It outlines the characteristics of saturated hydrocarbons (alkanes) and unsaturated hydrocarbons (alkenes and alkynes), detailing how to determine their structural formulas and naming based on carbon chains. Key points include the significance of prefixes for carbon counts and the impact of substituent groups on boiling points.