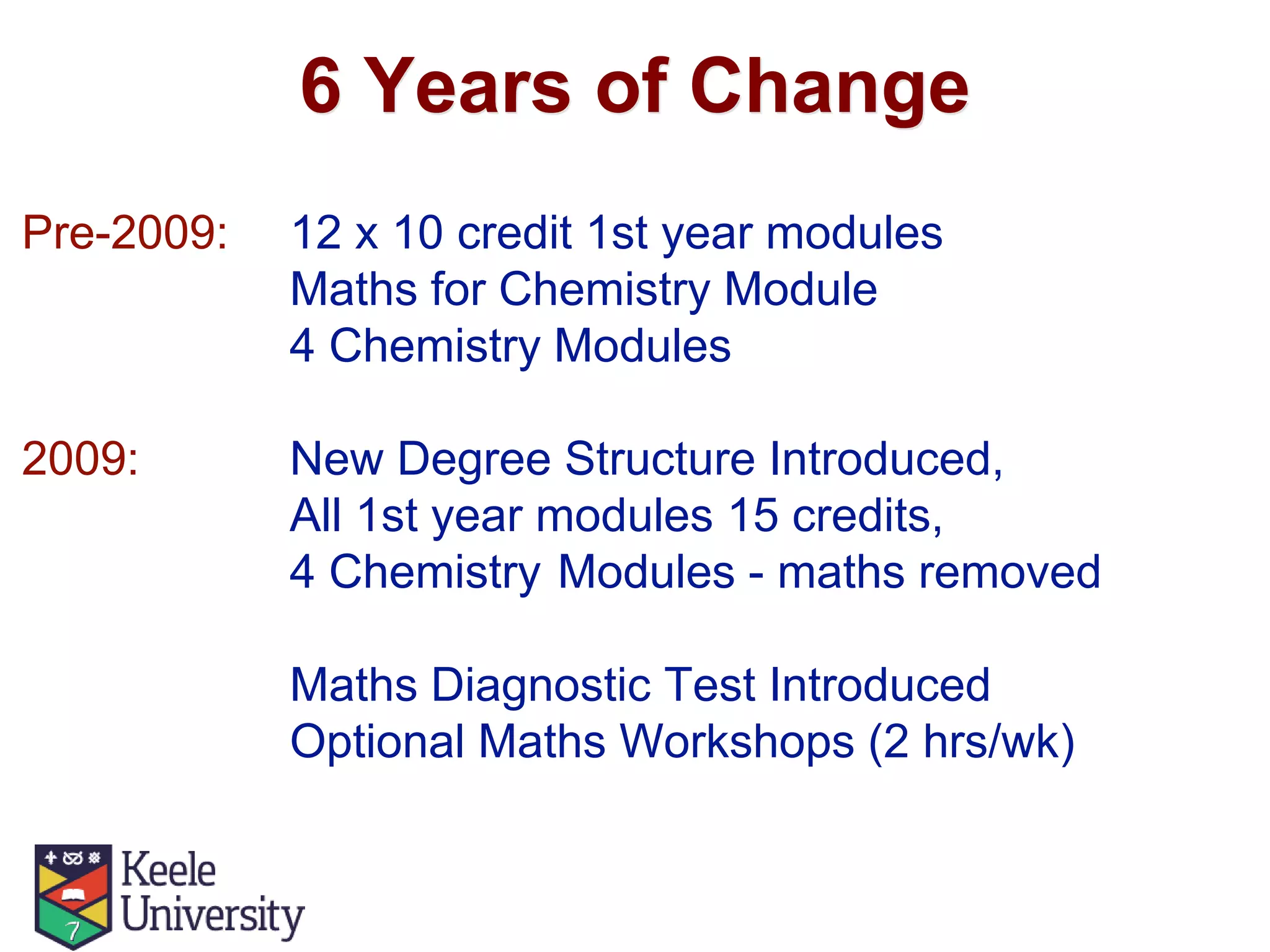
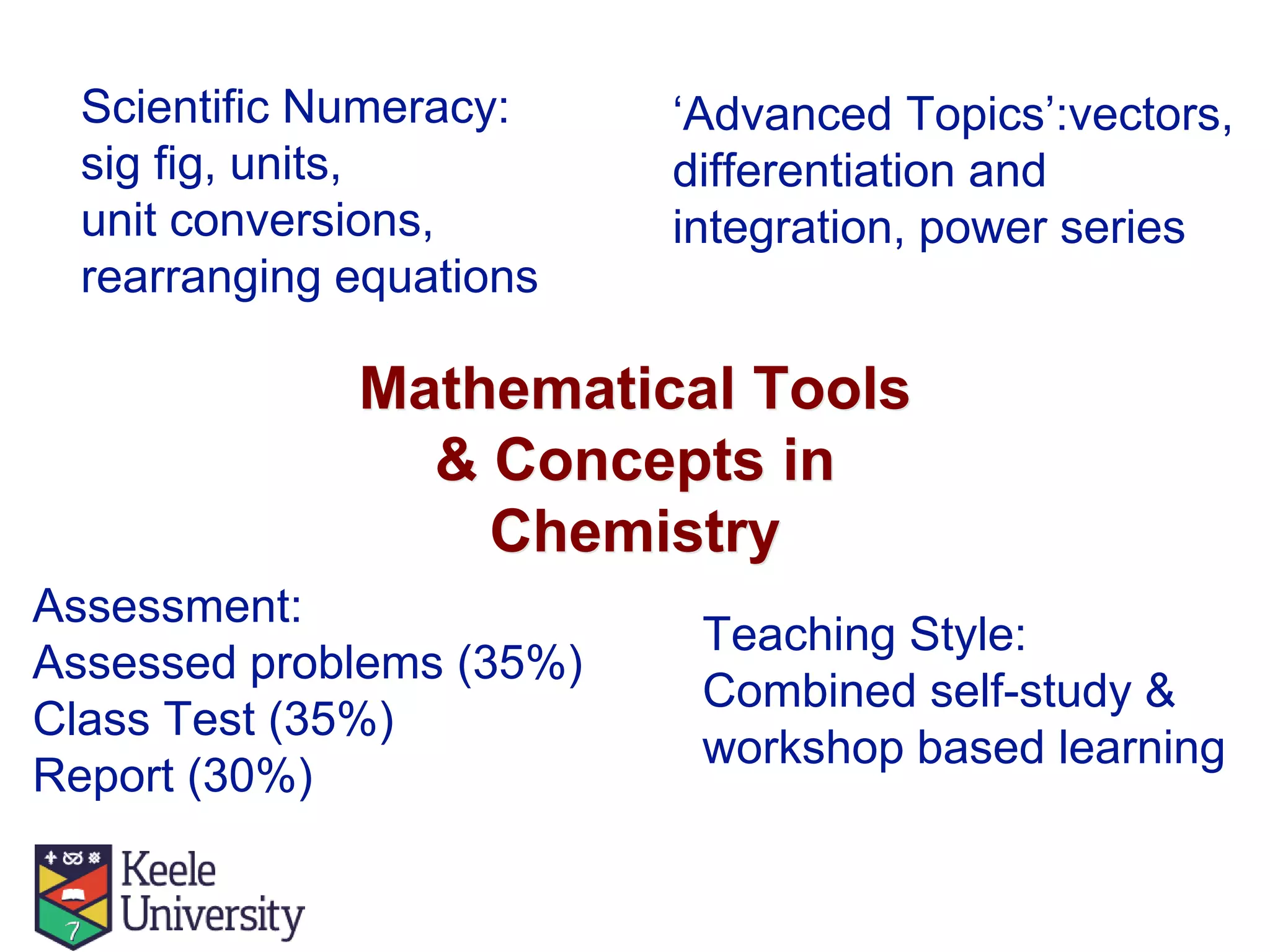
The document outlines the evolution of the Mathematics for Chemistry program at Keele University from 2009 to 2014, detailing curriculum changes and the reintroduction of mathematics into chemistry modules. It highlights the introduction of diagnostic tests, optional workshops, and emphasis on practical numeracy skills over advanced mathematical concepts. The curriculum was revised to include more laboratory hours and advanced mathematical topics, integrated with a combination of self-study and workshop-based learning.