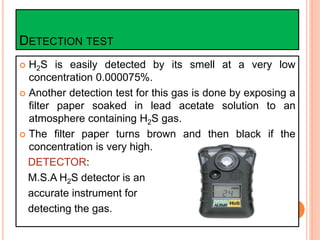
The document presents an overview of hydrogen sulfide (H2S), a highly toxic gas produced from decomposing organic materials and sulfur compounds. It details its properties, sources, health hazards, and the importance of detection and protection measures in environments where H2S may be present. Several historical accidents linked to H2S exposure underscore the critical need for safety protocols in mining operations.