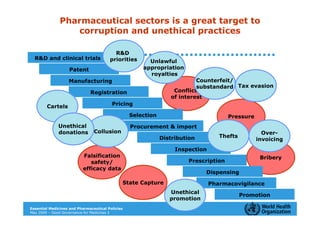
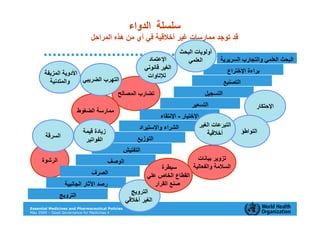
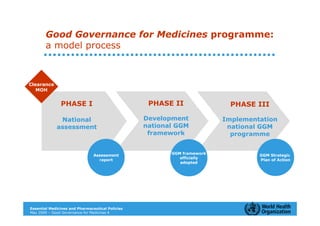
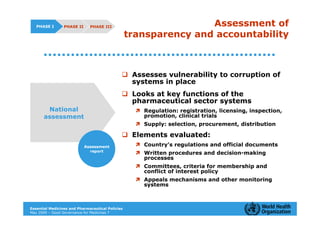
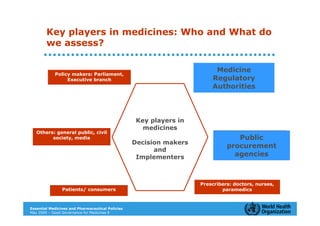
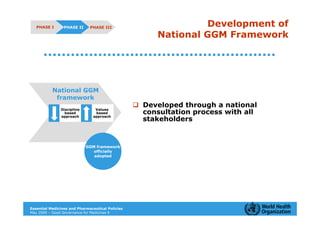
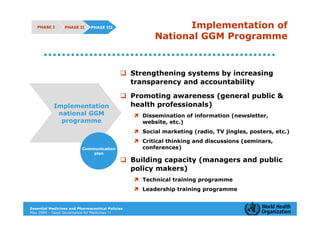
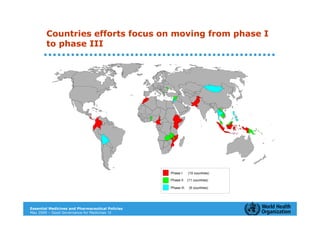
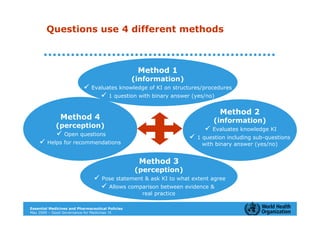
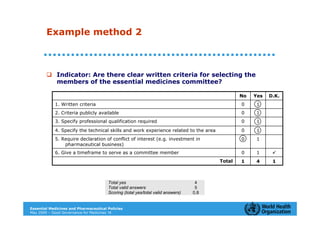
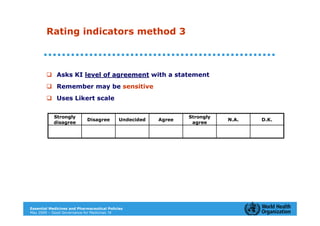
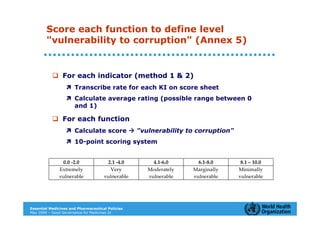
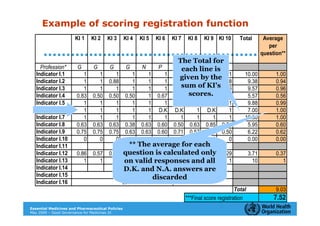
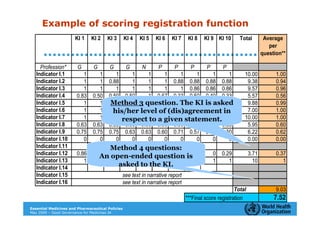
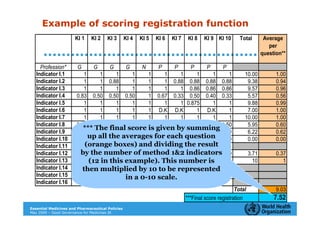
The document outlines the governance assessment methods and applications of governance data in policy-making related to the WHO's Good Governance for Medicines (GGM) programme, which aims to address corruption and unethical practices in the pharmaceutical sector. It details the phases of program assessment, the development of national frameworks, transparency assessments, and recommendations for enhancing accountability within public pharmaceutical sectors. Moreover, it emphasizes the importance of ethical practices, collaboration, and effective communication in implementing good governance strategies.