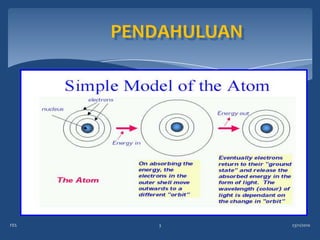
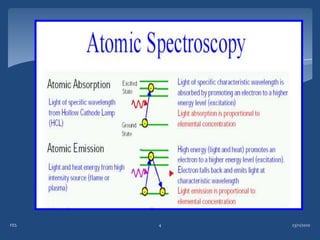
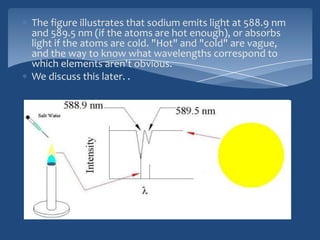
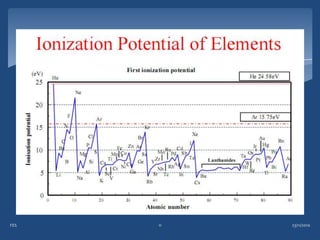
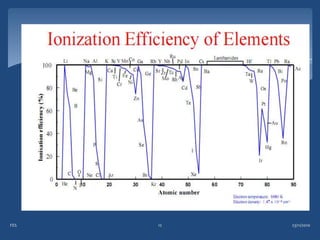
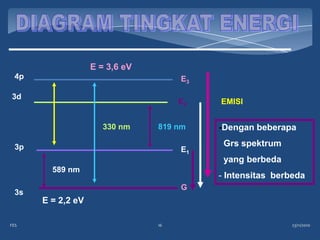
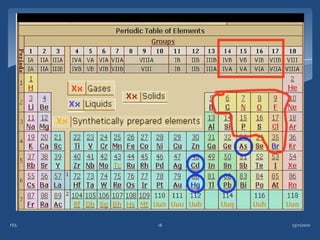
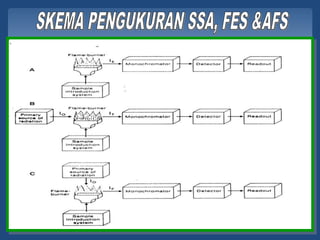
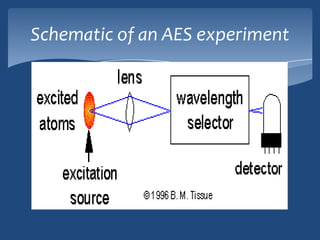
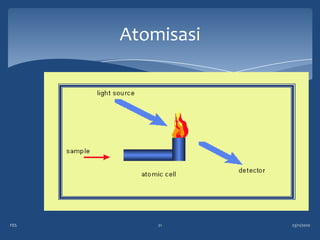
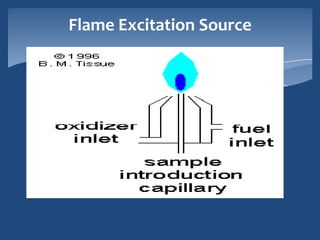
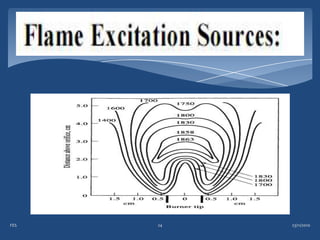
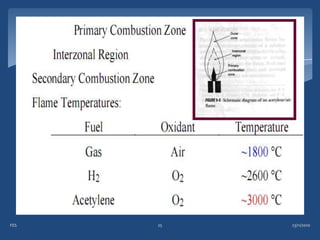
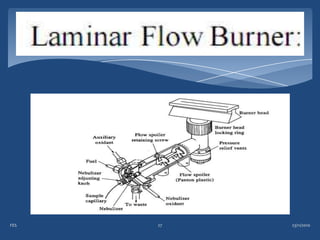
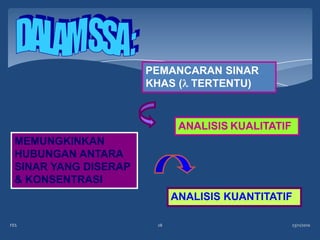
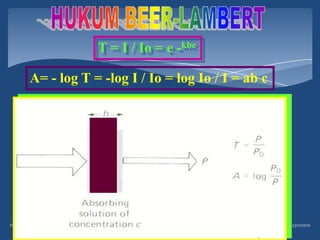
Atomic emission spectroscopy (AES) uses quantitative measurement of optical emission from excited atoms to determine analyte concentration. Analyte atoms are aspirated into an excitation source like a flame, discharge, or plasma which provides energy to promote atoms into high energy levels. As atoms decay back to lower levels, they emit light of wavelengths specific to each element. AES can simultaneously detect multiple elements and is used for elemental analysis, identification, quantification, and sometimes speciation of samples.