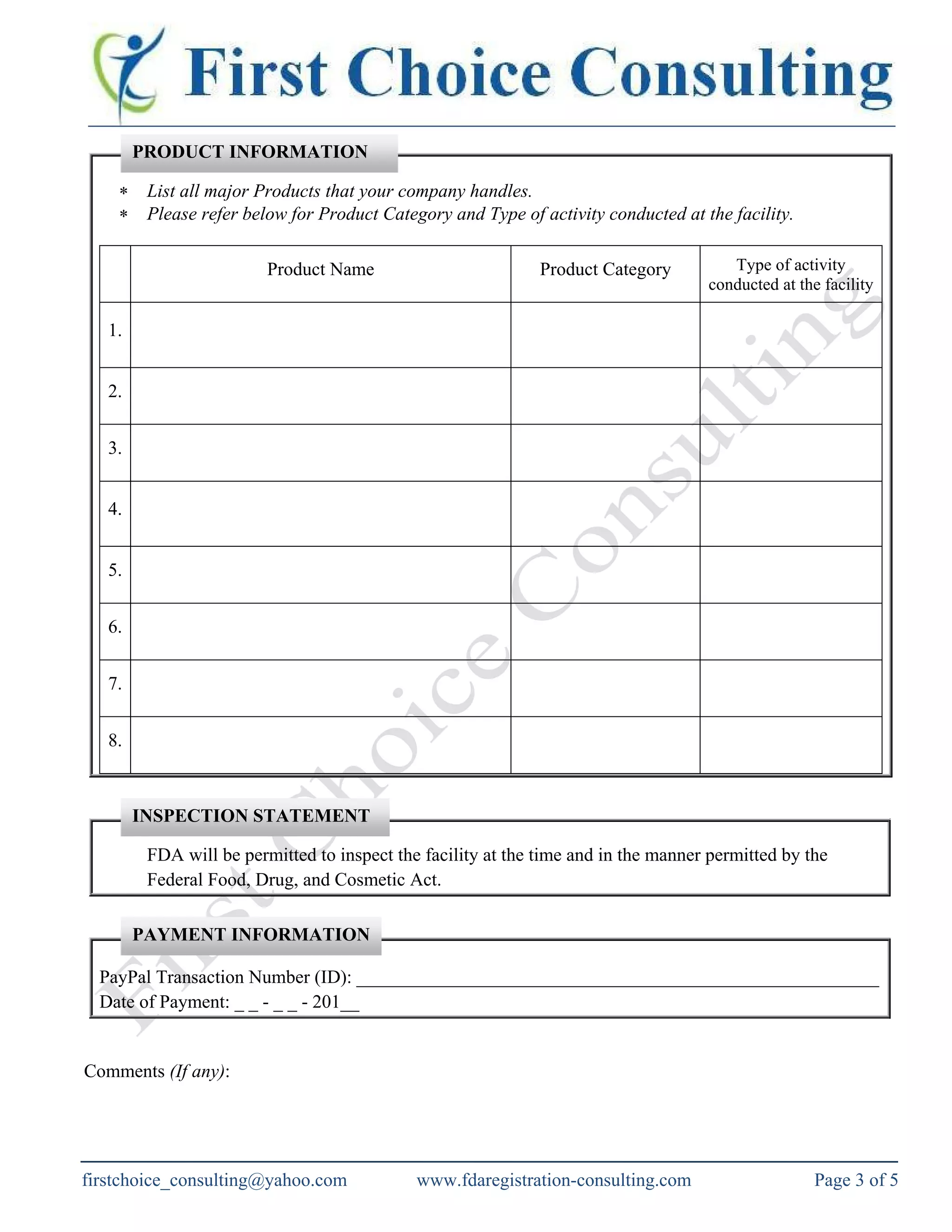
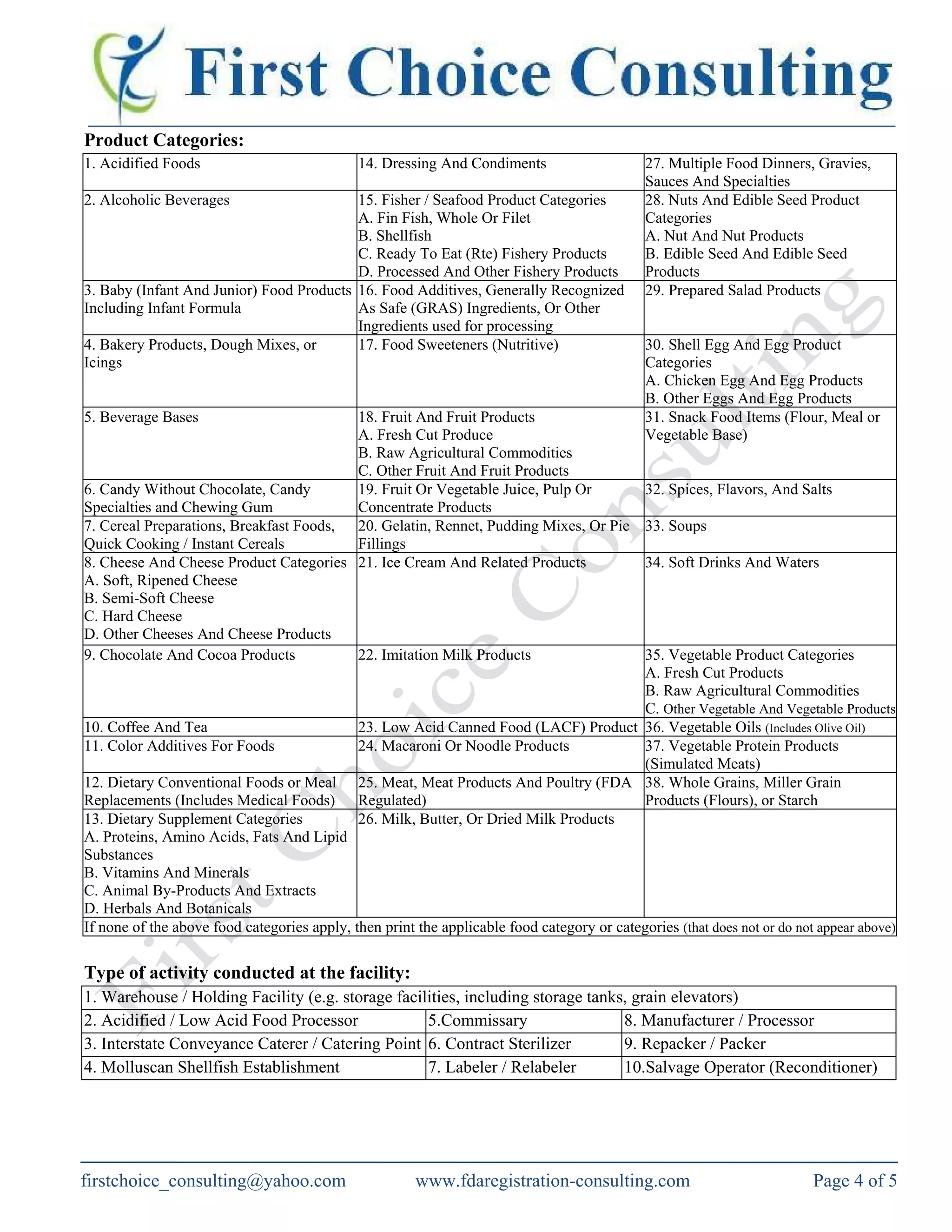
The document outlines the requirements for food facility registration with the US FDA under the Bioterrorism Act for both domestic and foreign facilities. It explains the need for foreign facilities to designate a US agent, details the registration process, and provides a food facility registration form. The document also offers consulting services to assist with FDA registration and compliance for various types of food businesses.