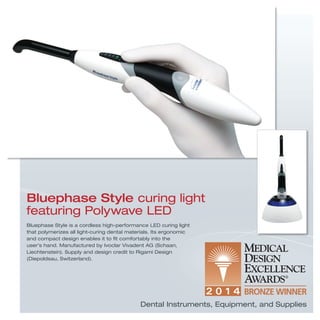
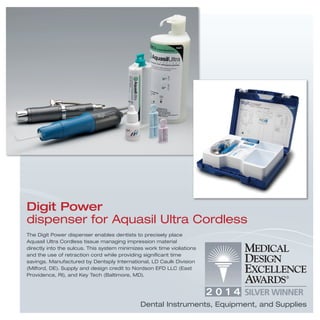
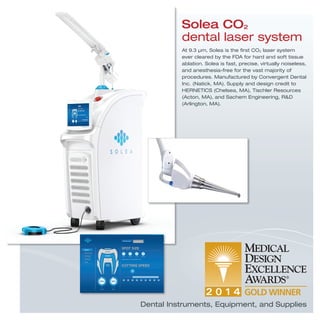
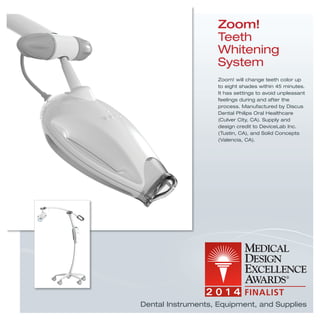
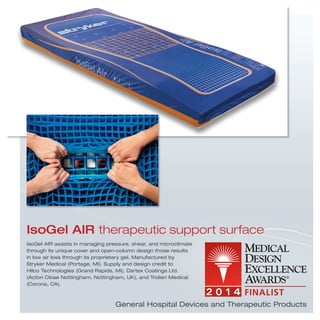
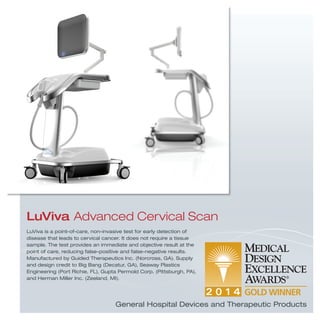
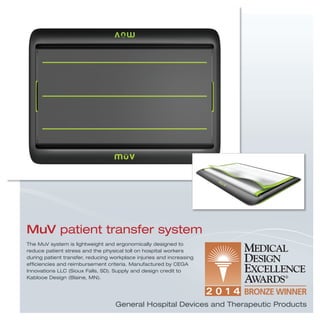
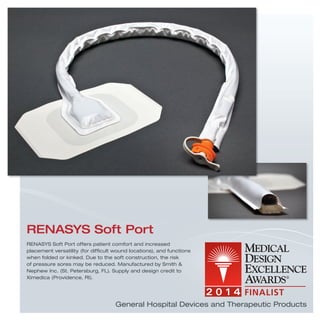
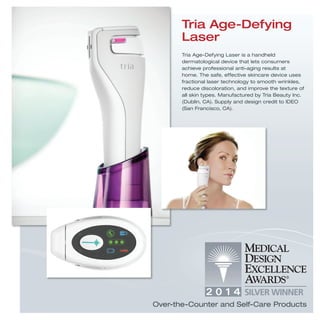
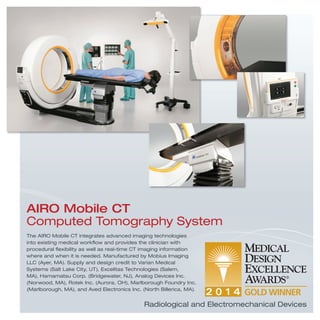
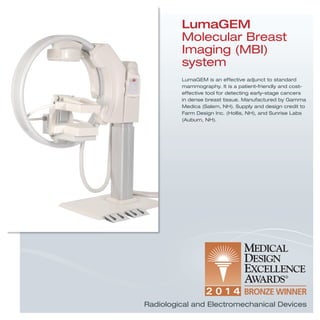
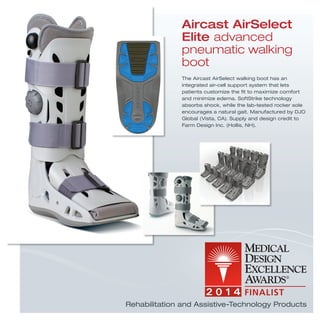
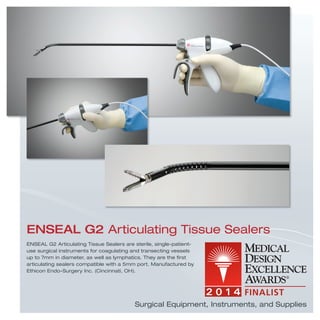
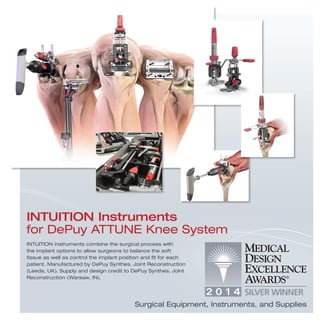
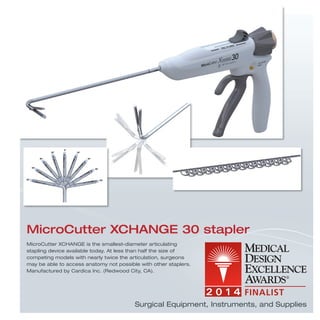
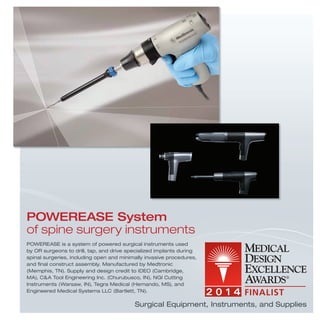
The document provides information on various medical products, including:
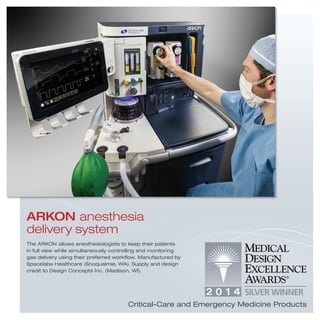
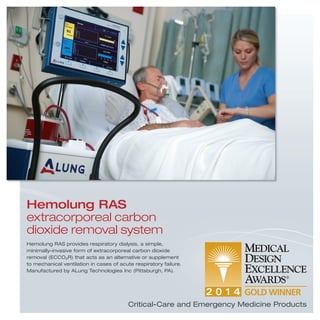
1) The ARKON anesthesia delivery system and Hemolung RAS extracorporeal carbon dioxide removal system which aid anesthesiologists and treat acute respiratory failure.
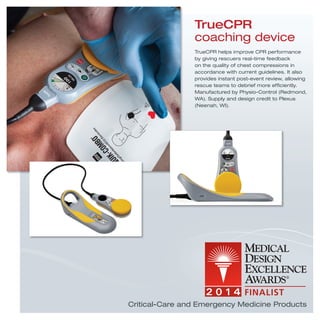
2) Isolibrium critical-care air support surface, RP-VITA remote presence telemedicine robot, and TrueCPR coaching device which help nurses care for patients, provide telemedicine consults, and improve CPR performance.
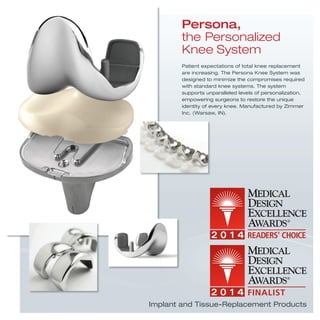
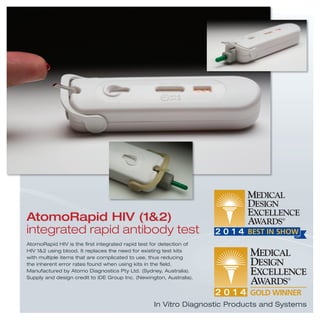
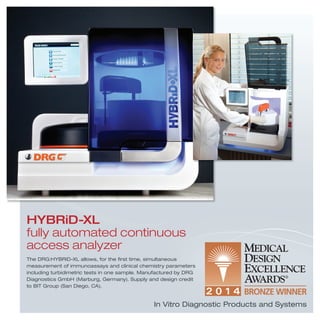
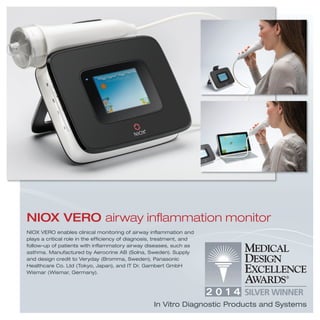
3) Additional devices, systems, products and tools that address a range of medical needs from dental care and drug delivery to diagnostics, implants, hospital equipment and more.