Enzymes are biological catalysts, usually proteins (some are RNA – ribozymes), that speed up chemical reactions in living organisms without being consumed in the process.
Key Features of Enzymes:
Catalysts: Increase the rate of biochemical reactions by lowering the activation energy.
Specificity: Each enzyme is highly specific to its substrate (the molecule it acts on).
Active Site: The region of the enzyme where the substrate binds and reaction occurs.
Reusable: They are not consumed in the reaction, so they can be used repeatedly.
Regulated: Their activity can be enhanced or inhibited, ensuring metabolic control.
Efficient: Reactions that would take years can occur in milliseconds with enzymes.
Structure:
Mostly proteins with a unique 3D structure.
Some require cofactors (metal ions like Zn²⁺, Mg²⁺) or coenzymes (organic molecules like NAD⁺, FAD, vitamins).
Types (based on reaction):
Oxidoreductases – oxidation/reduction reactions (e.g., dehydrogenases).
Transferases – transfer functional groups (e.g., kinases).
Hydrolases – hydrolysis reactions (e.g., lipases, proteases).
Lyases – add/remove groups without hydrolysis (e.g., decarboxylases).
Isomerases – rearrange atoms within a molecule (e.g., mutases).
Ligases – join molecules using ATP (e.g., DNA ligase).
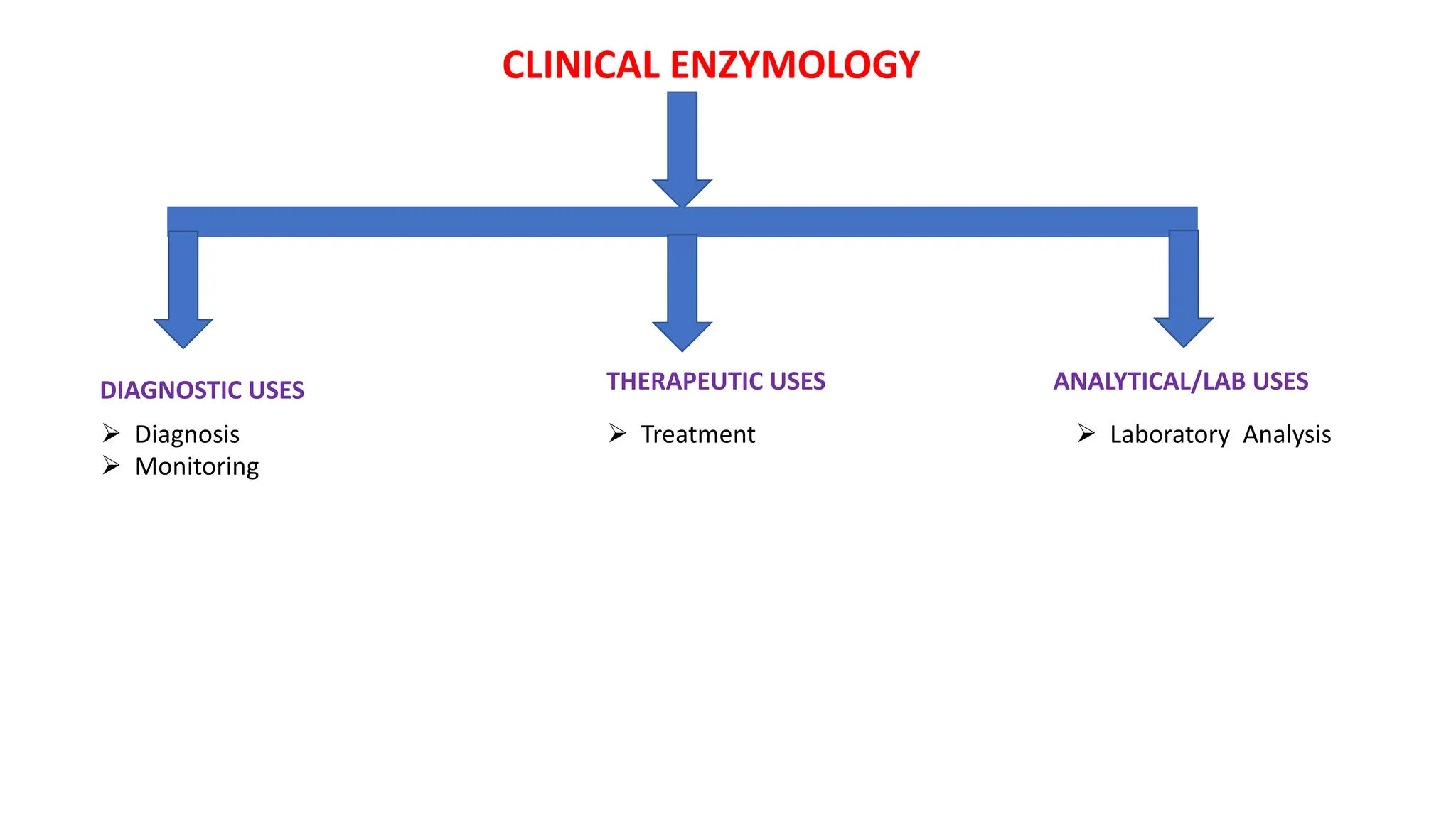
Clinical Importance:
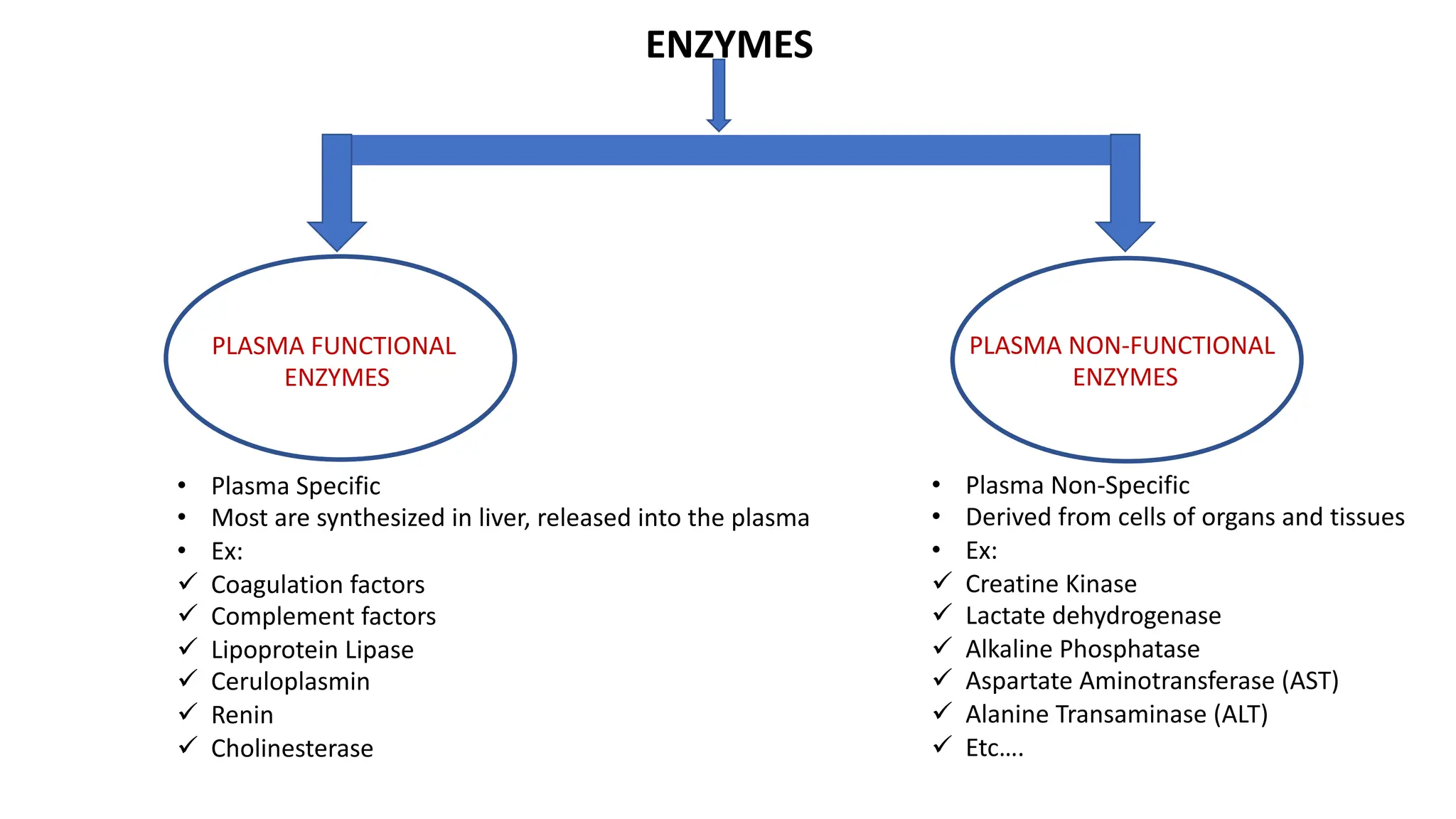
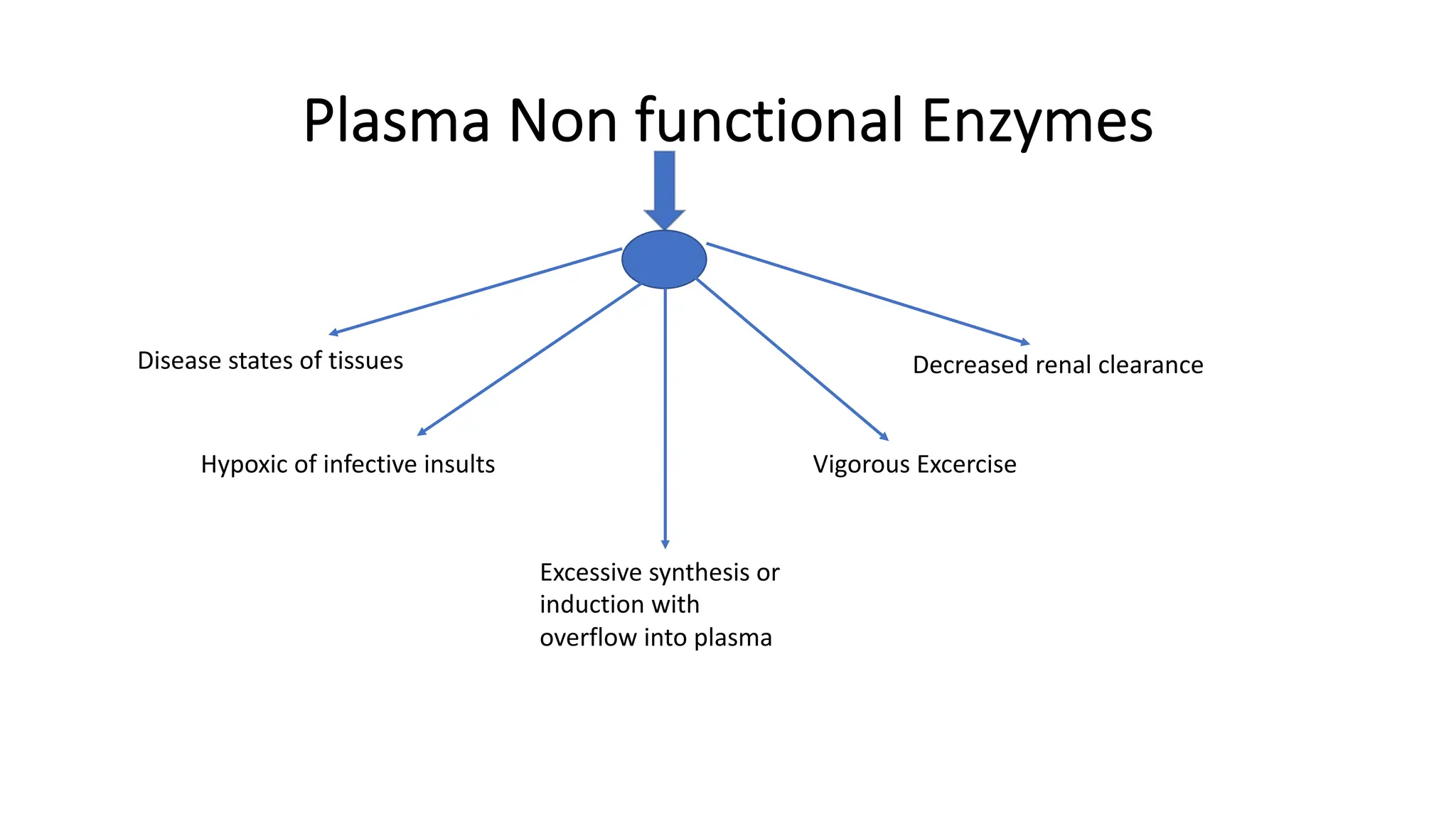
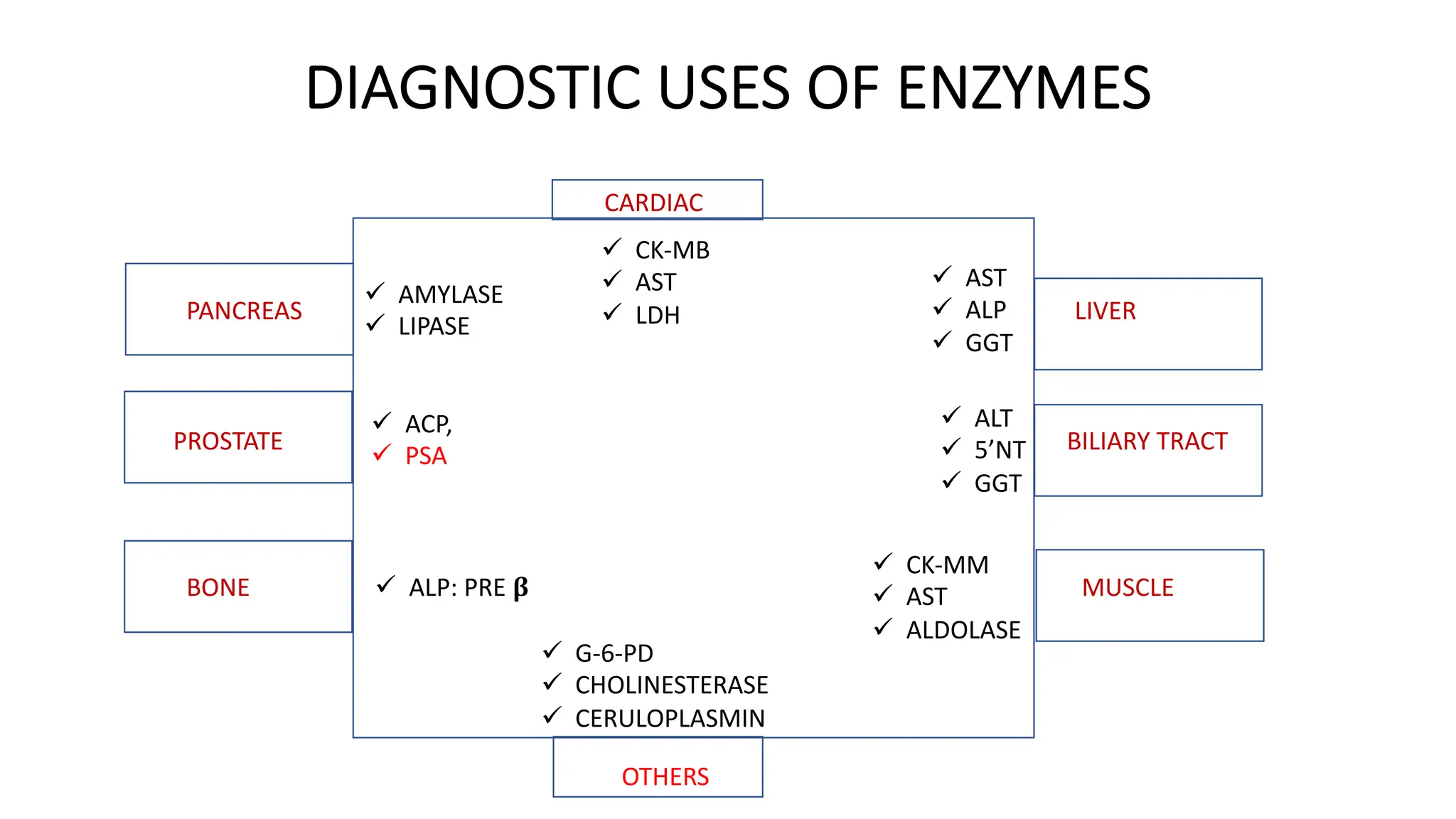
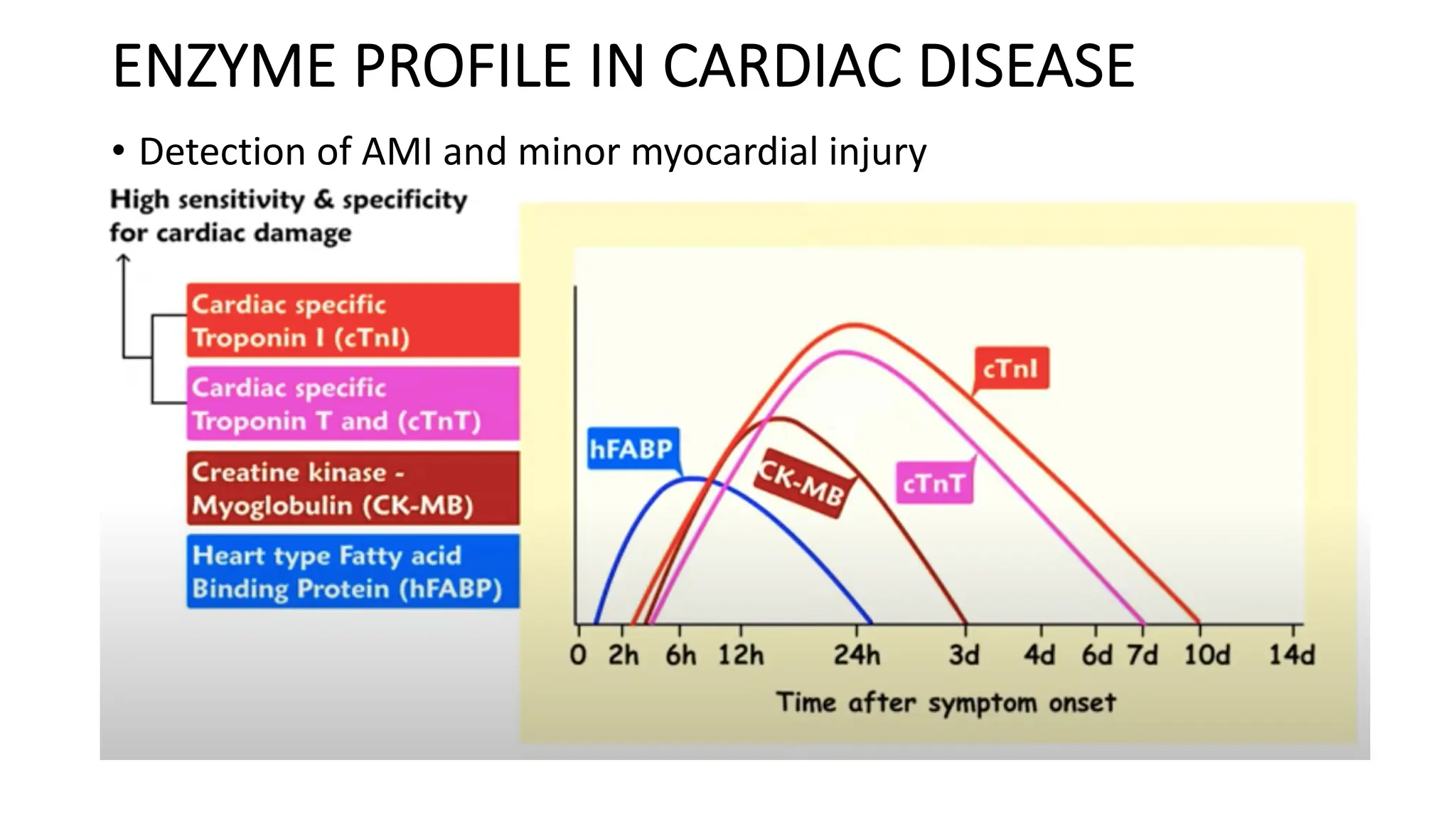
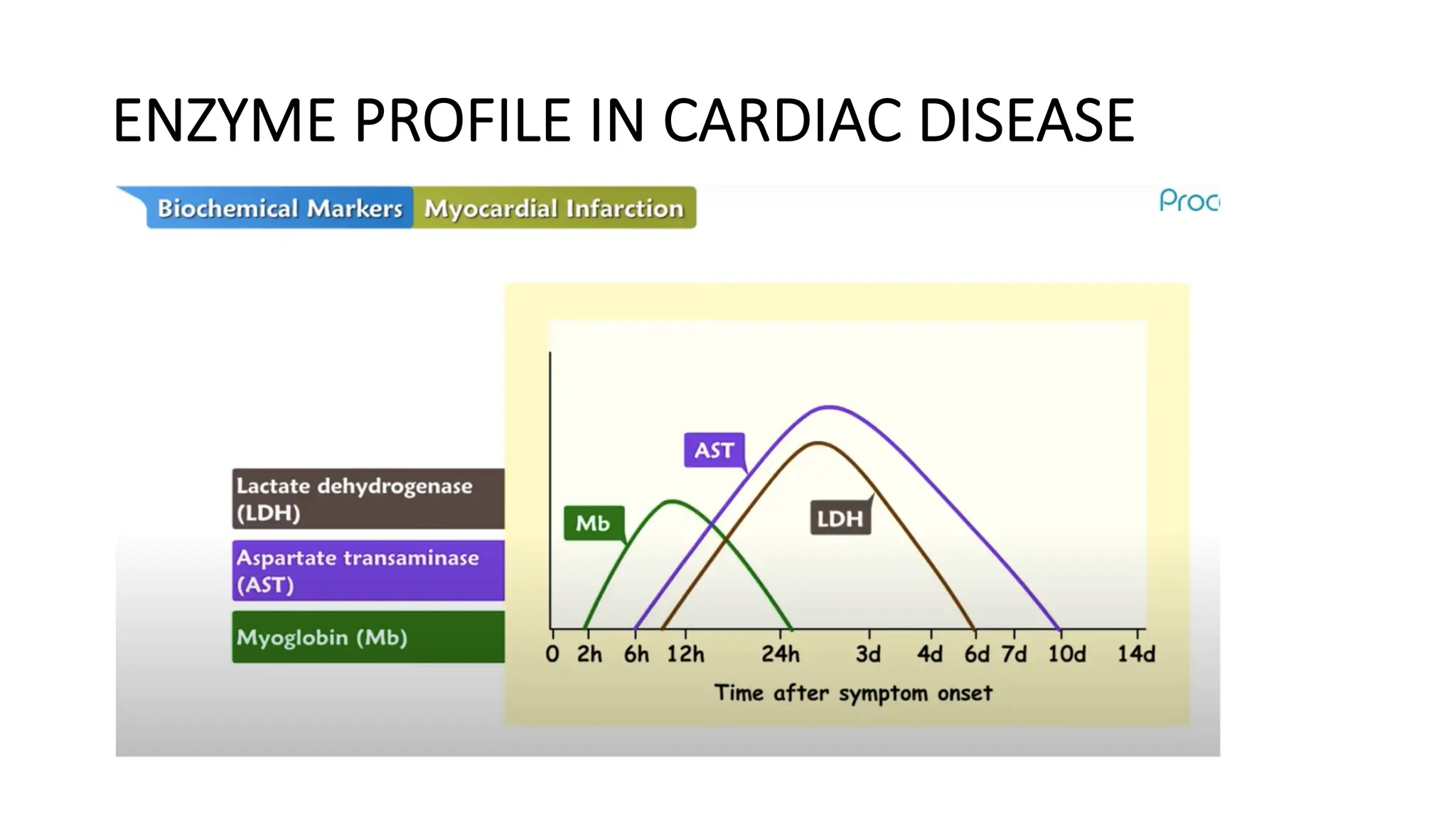
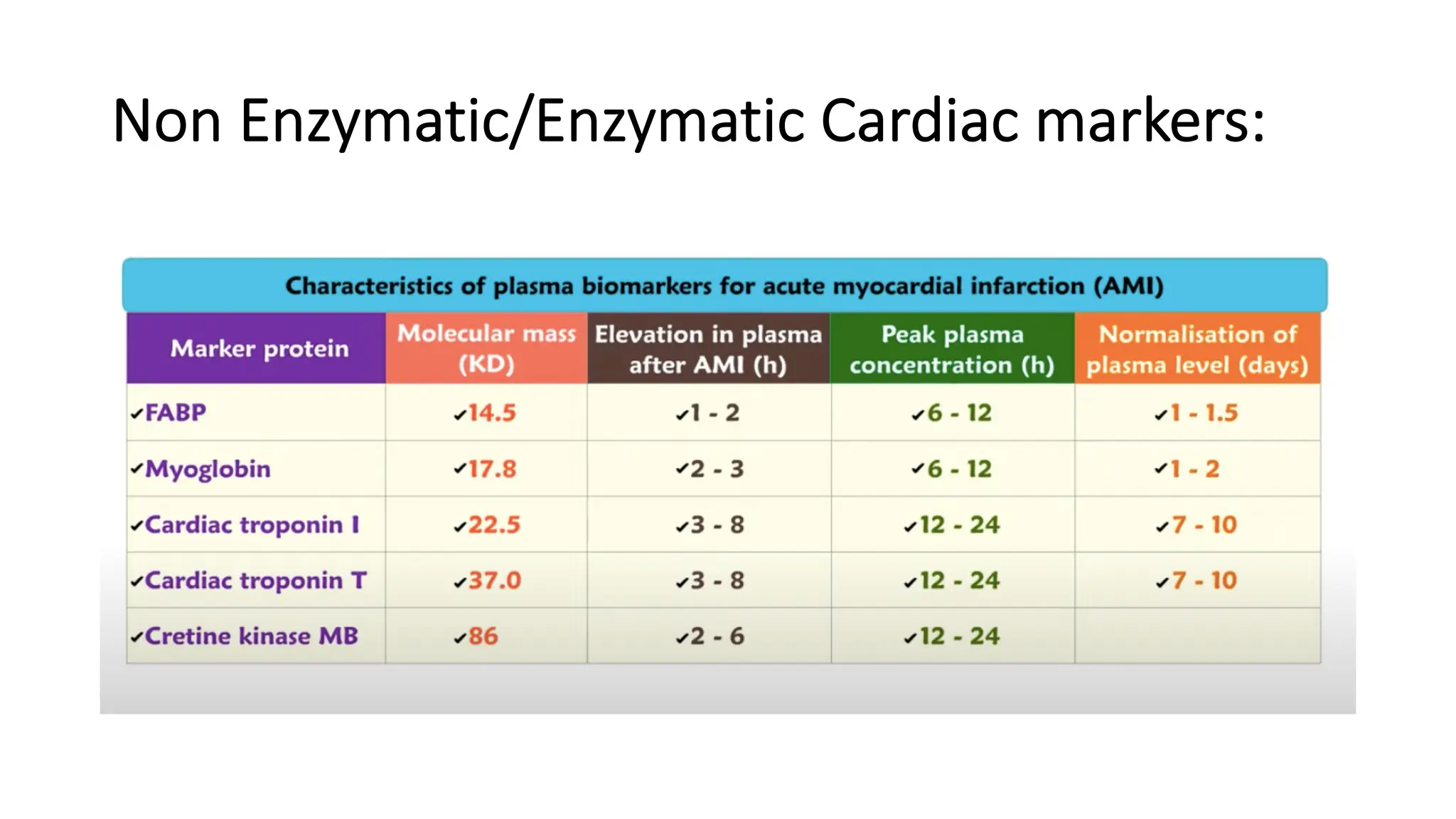
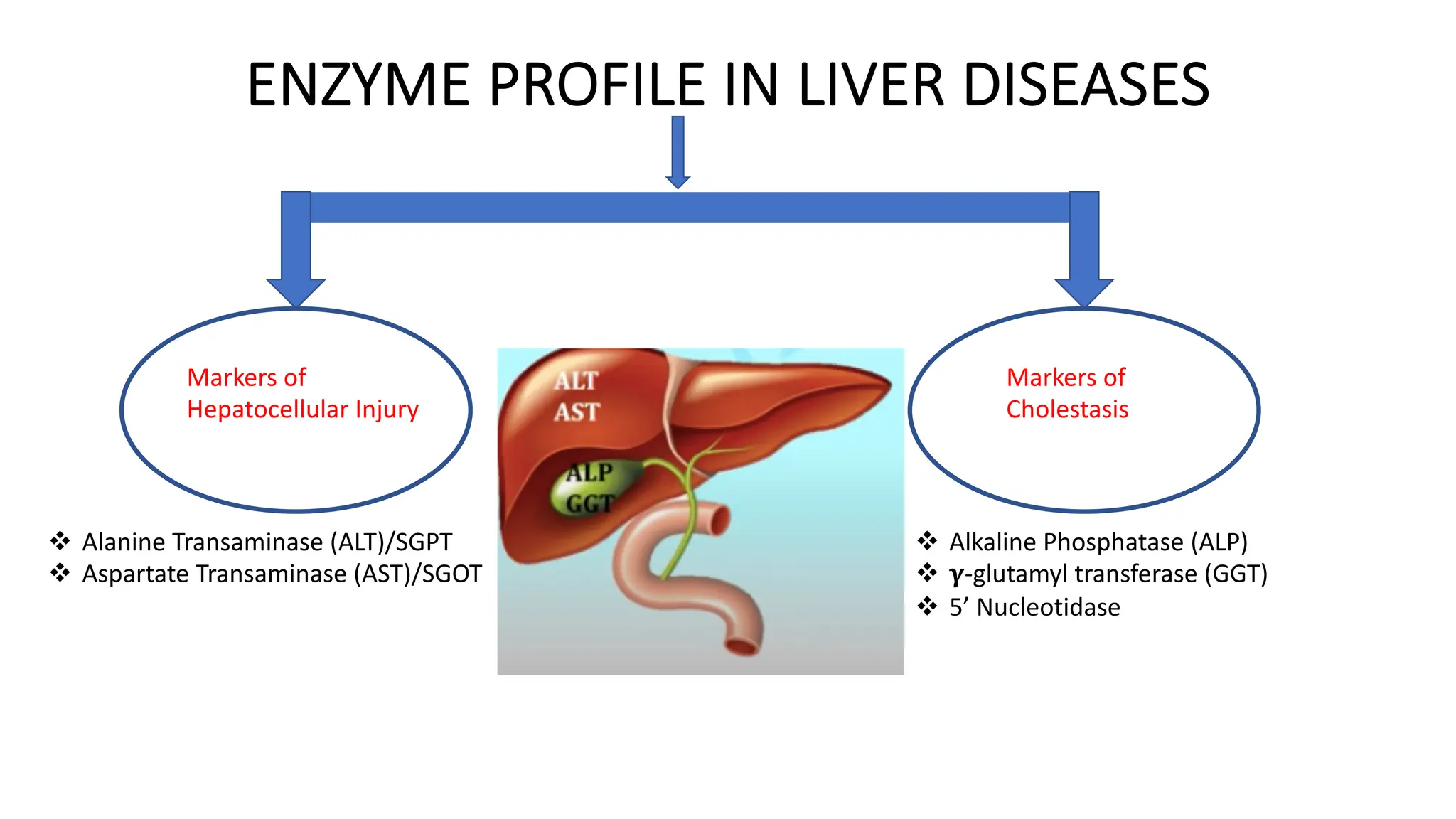
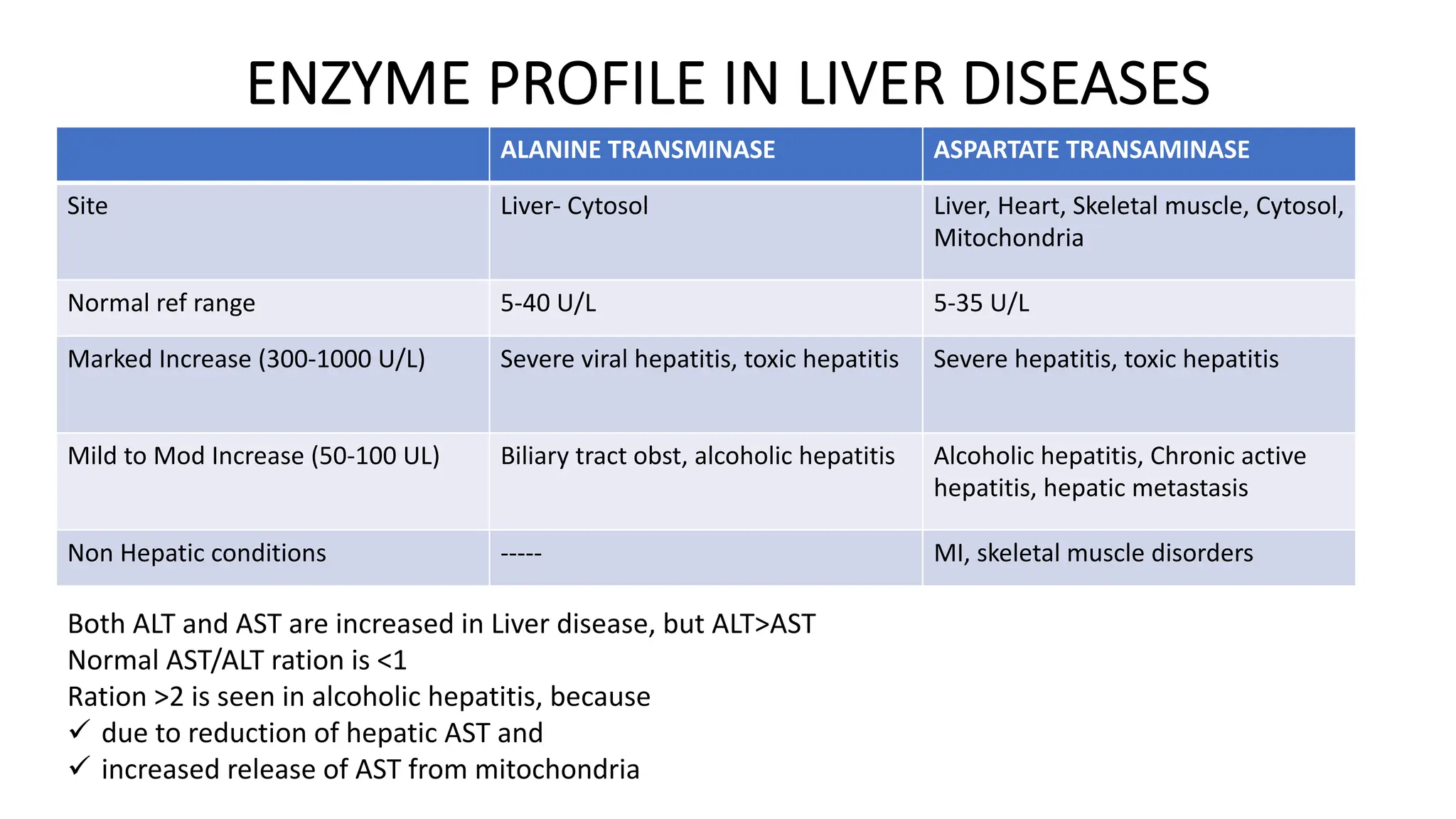
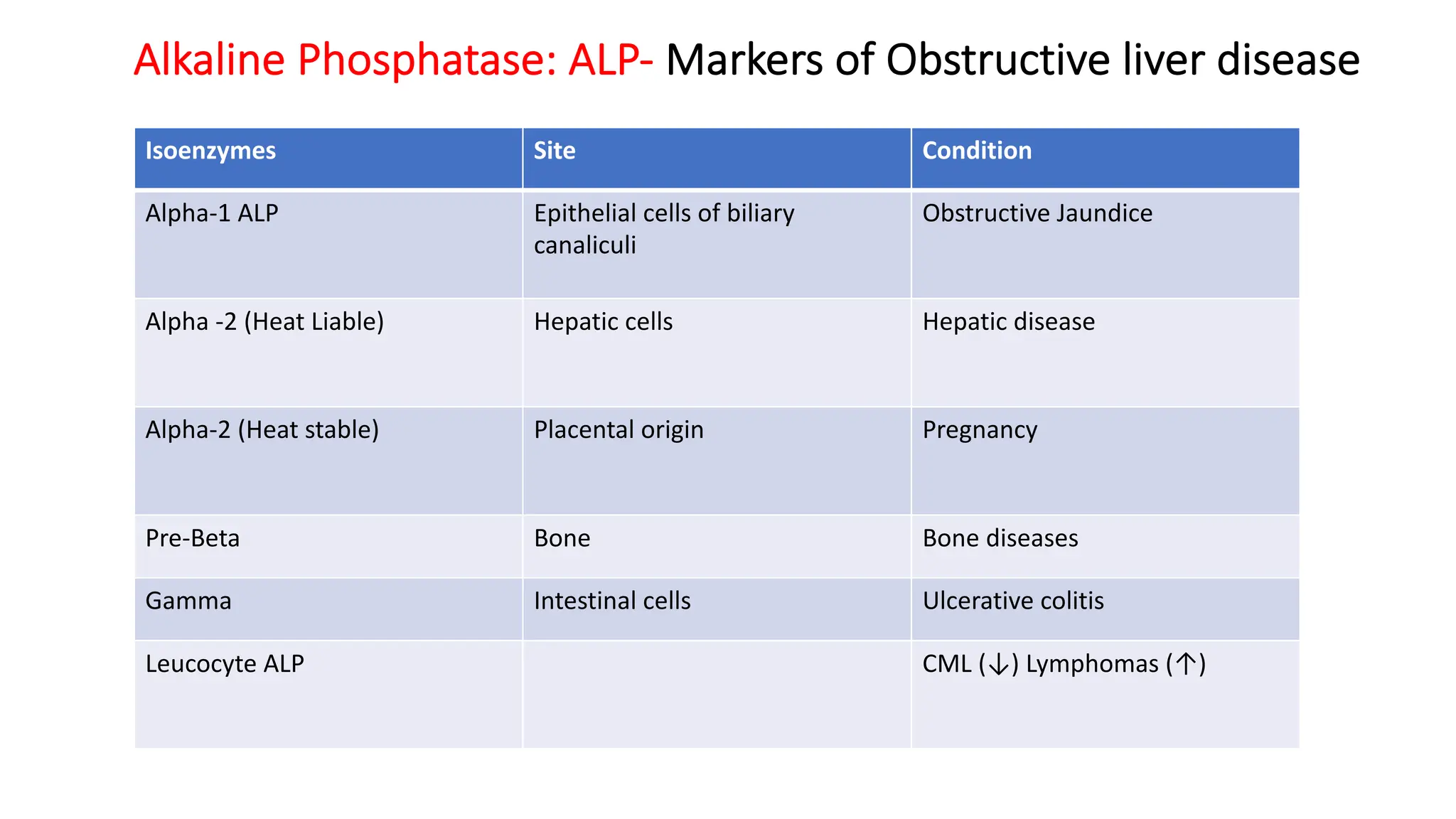
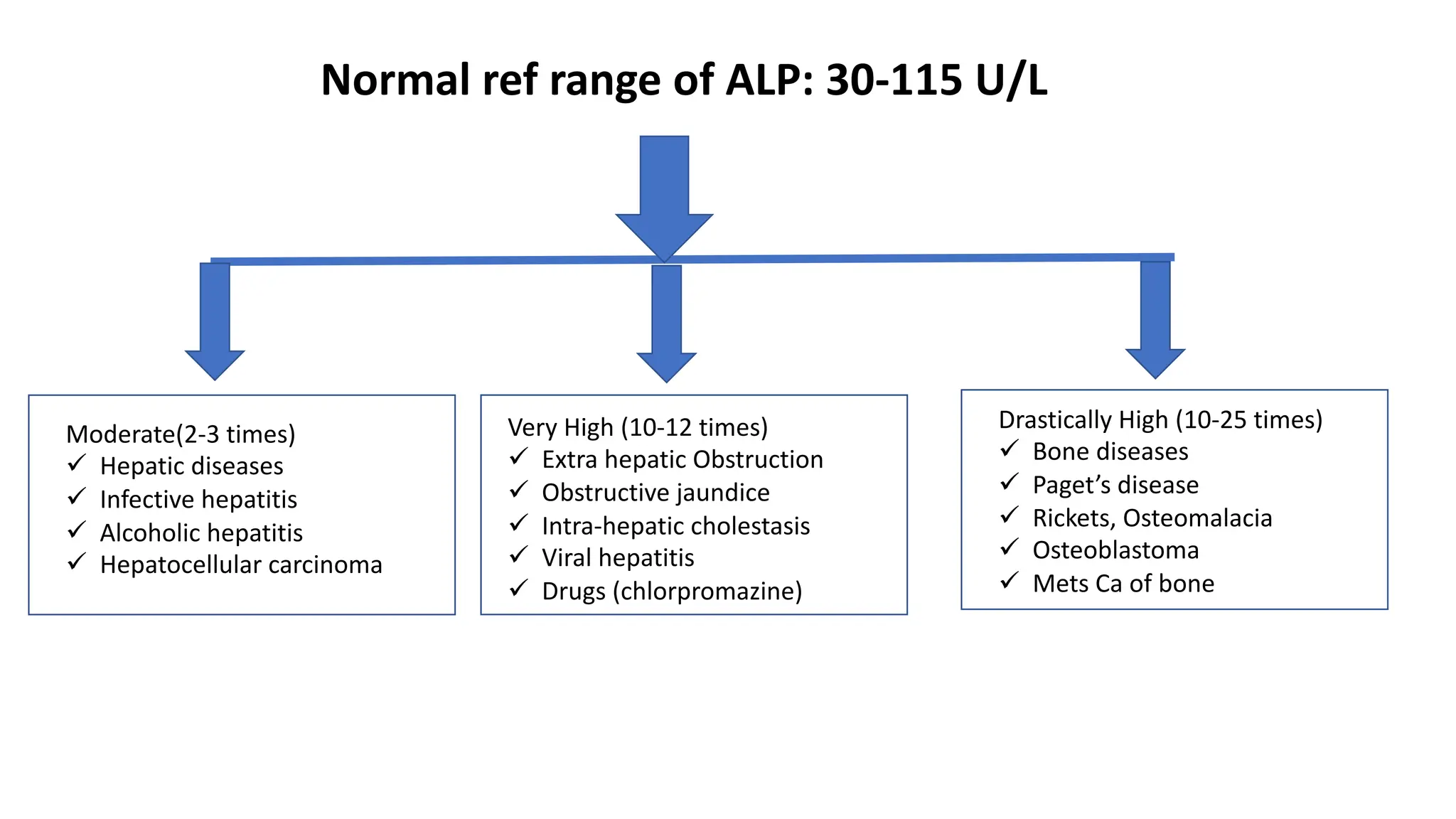
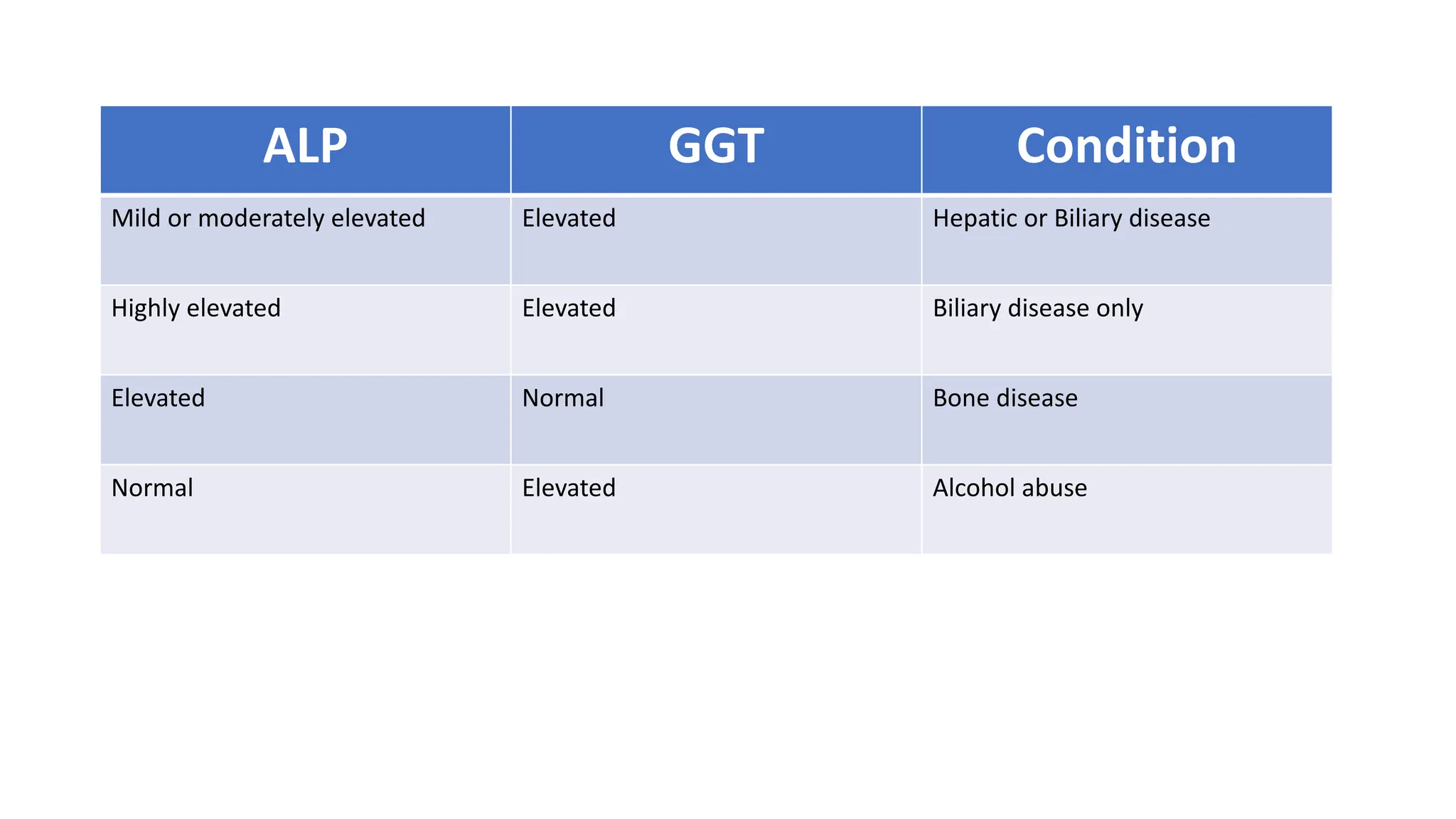
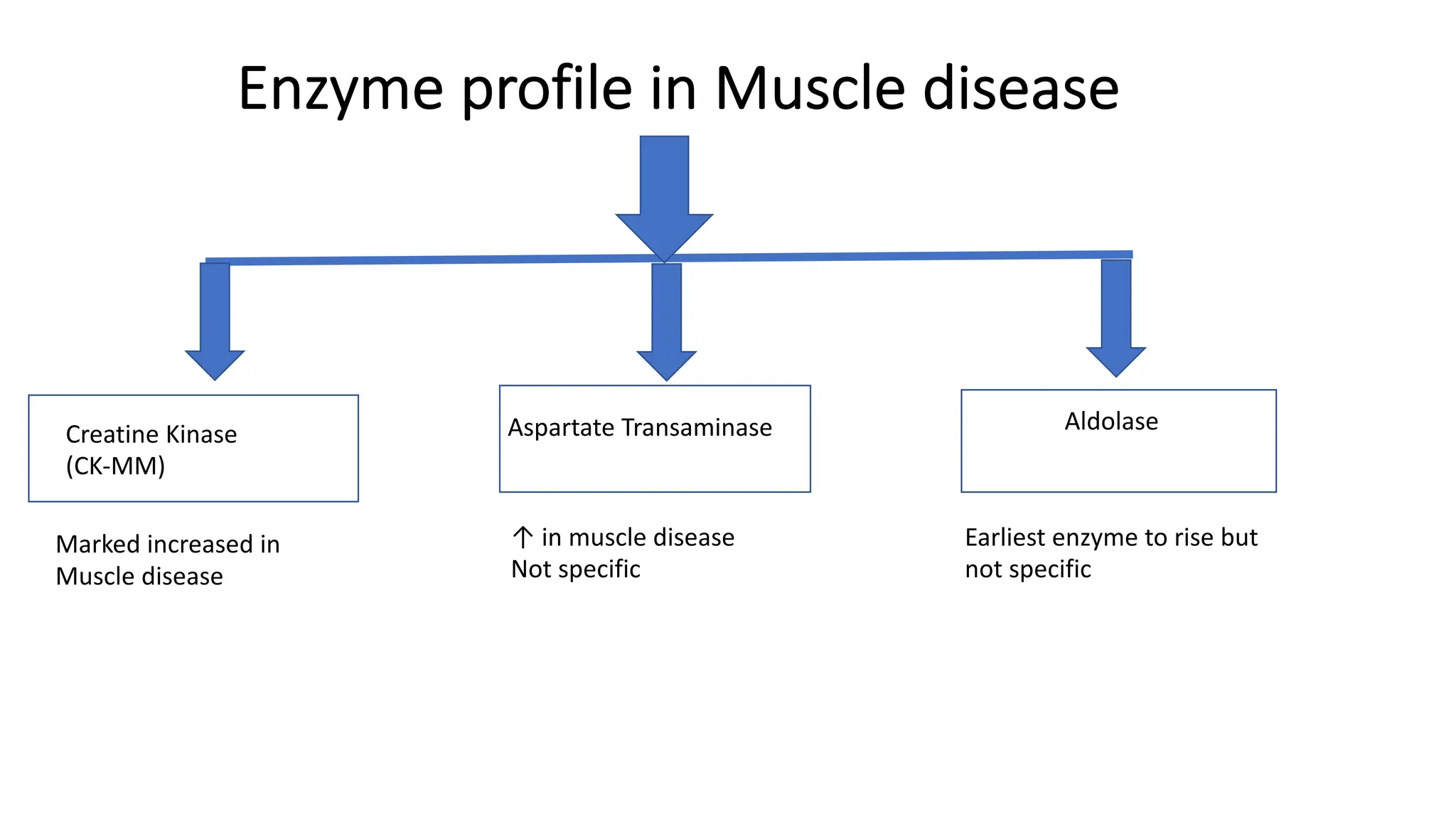
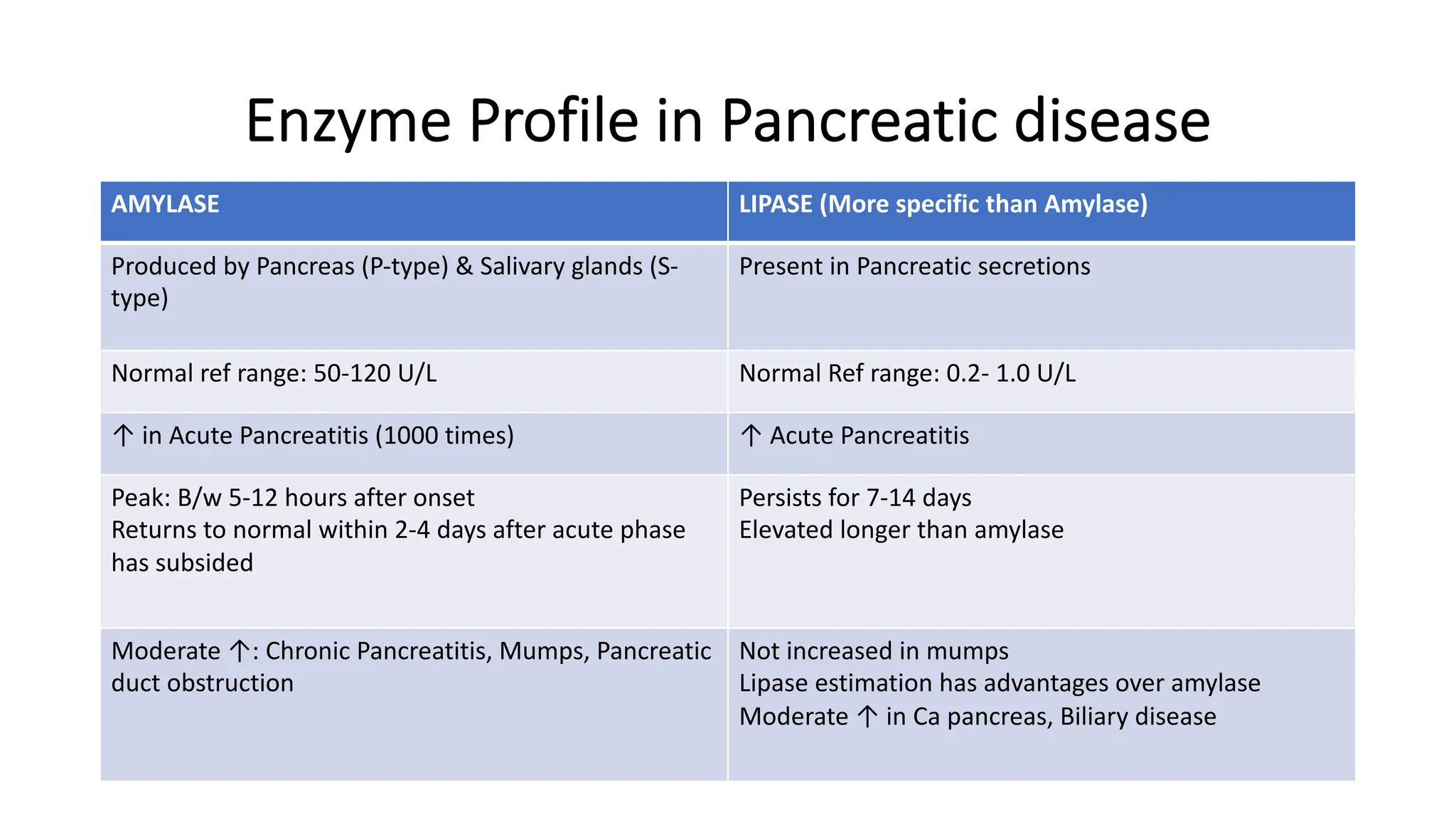
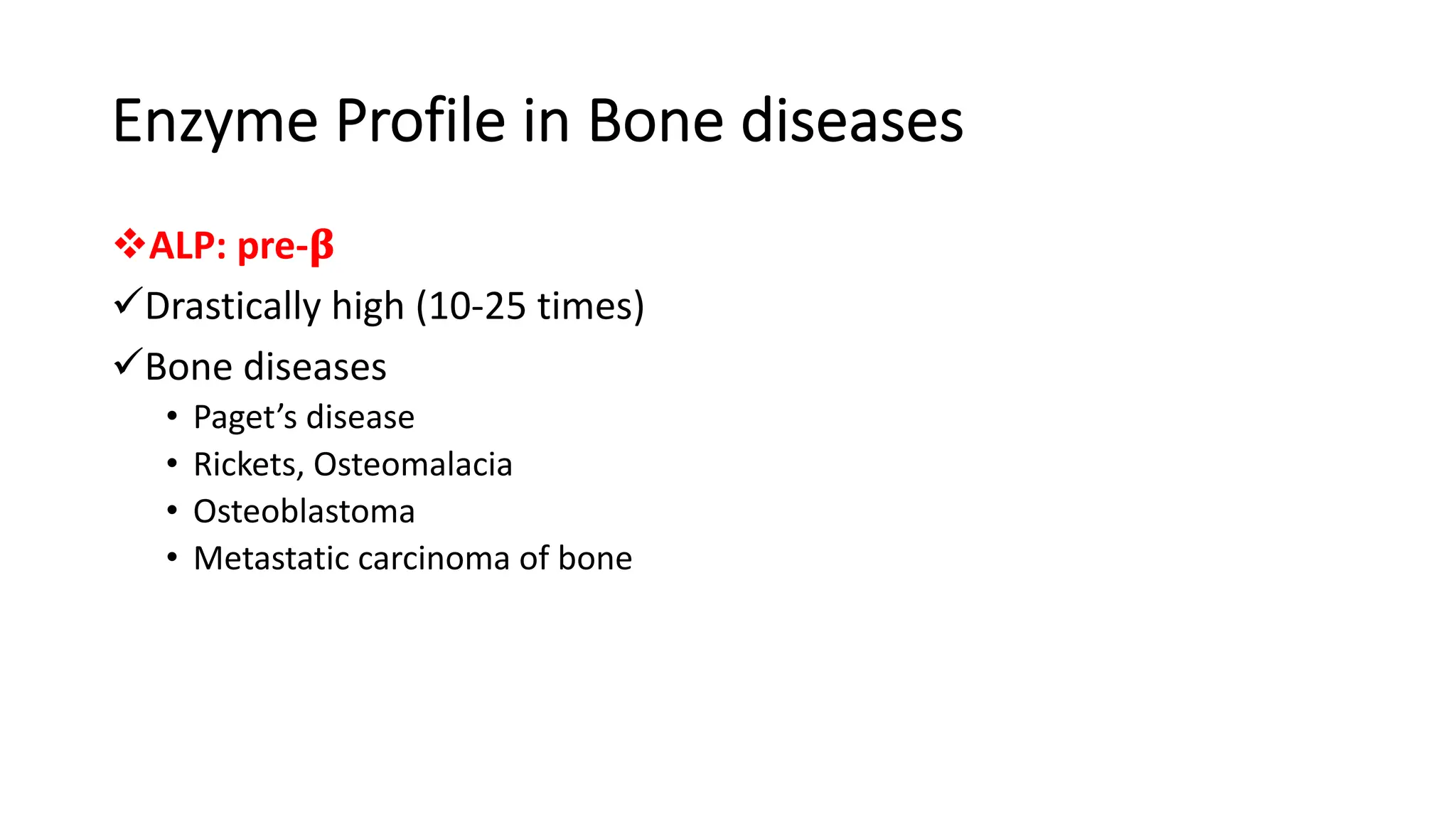
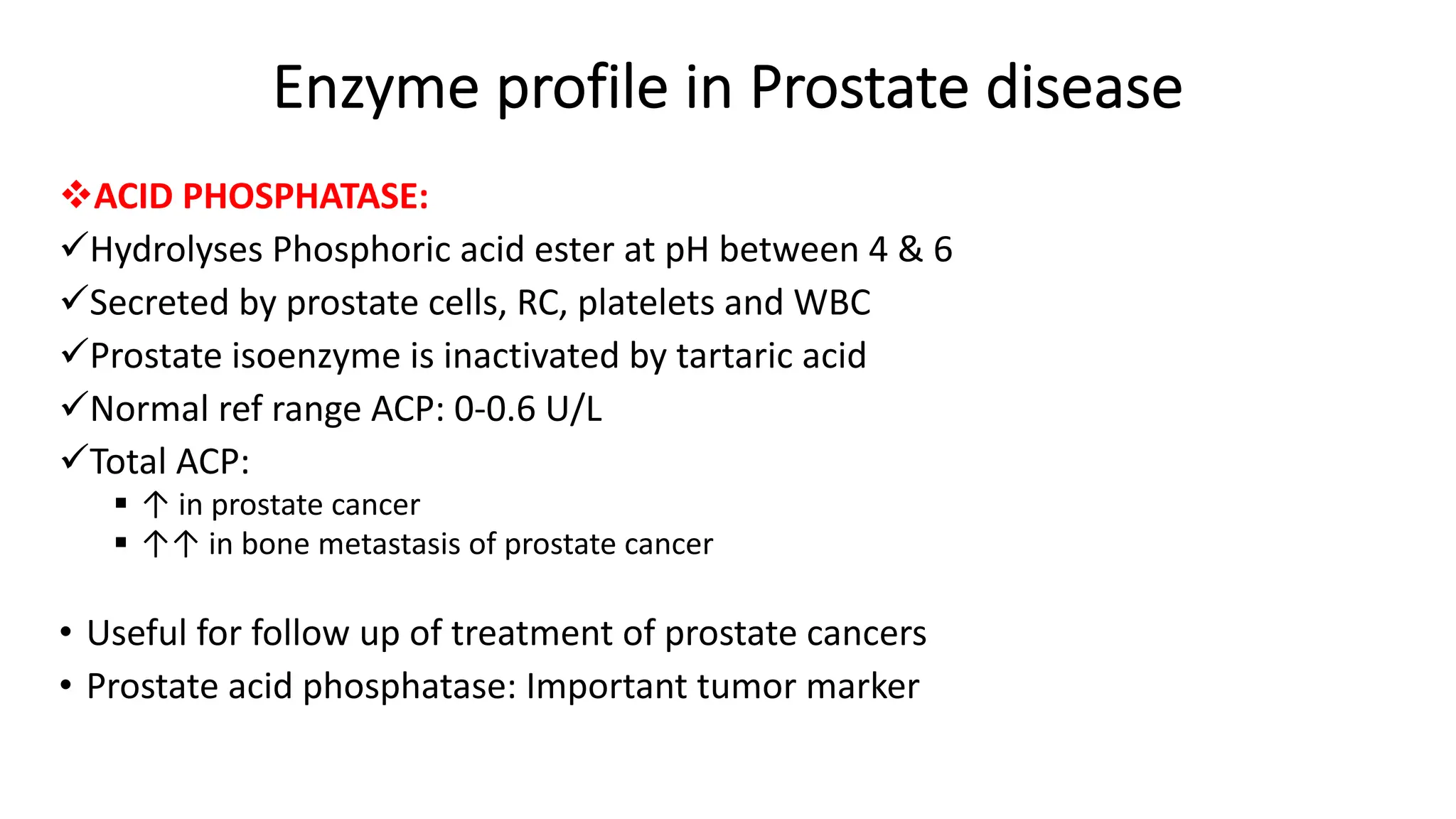
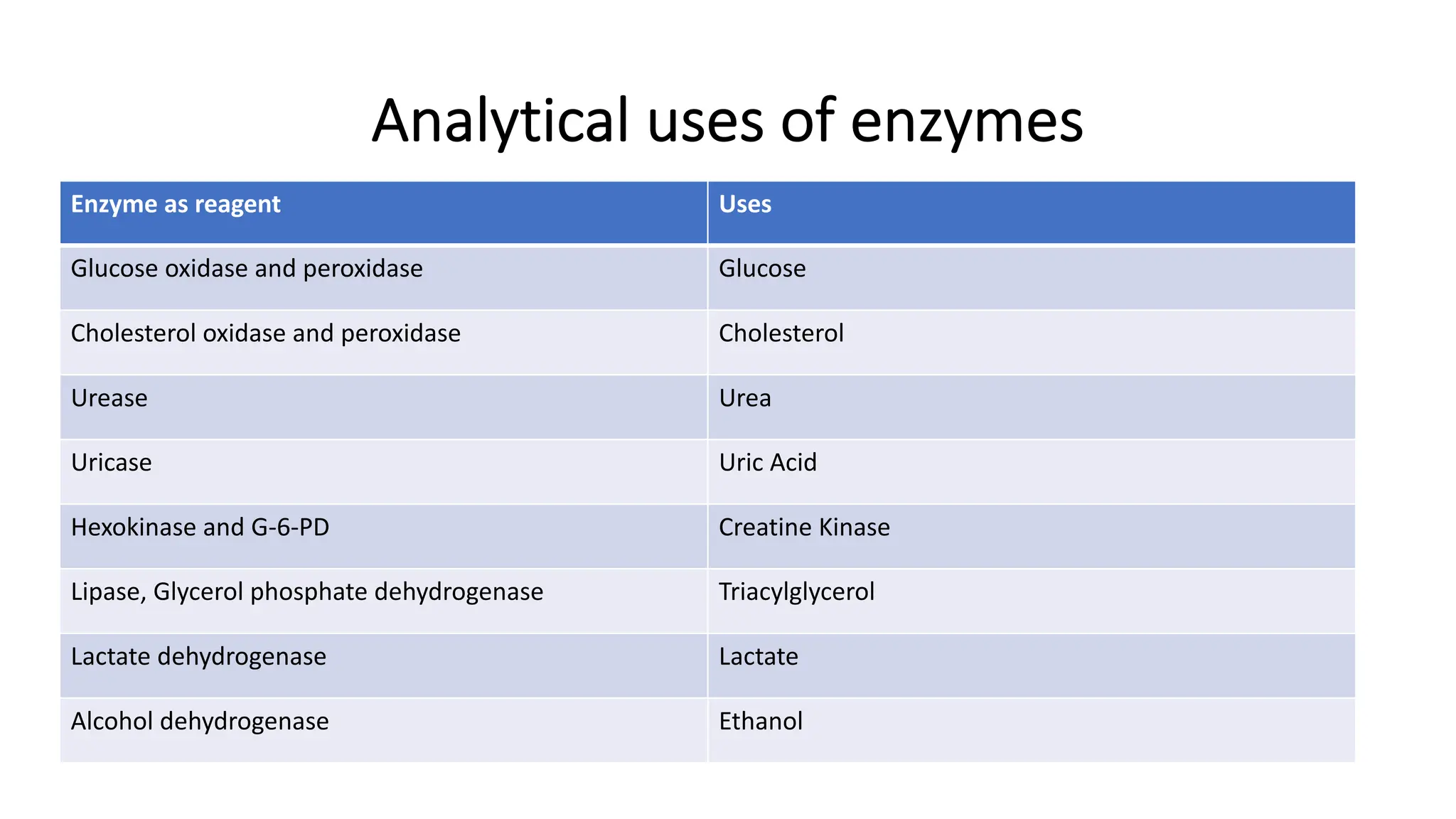
Enzyme levels in blood are used as biomarkers for diseases (e.g., elevated ALT/AST in liver disease, CK-MB in myocardial infarction).
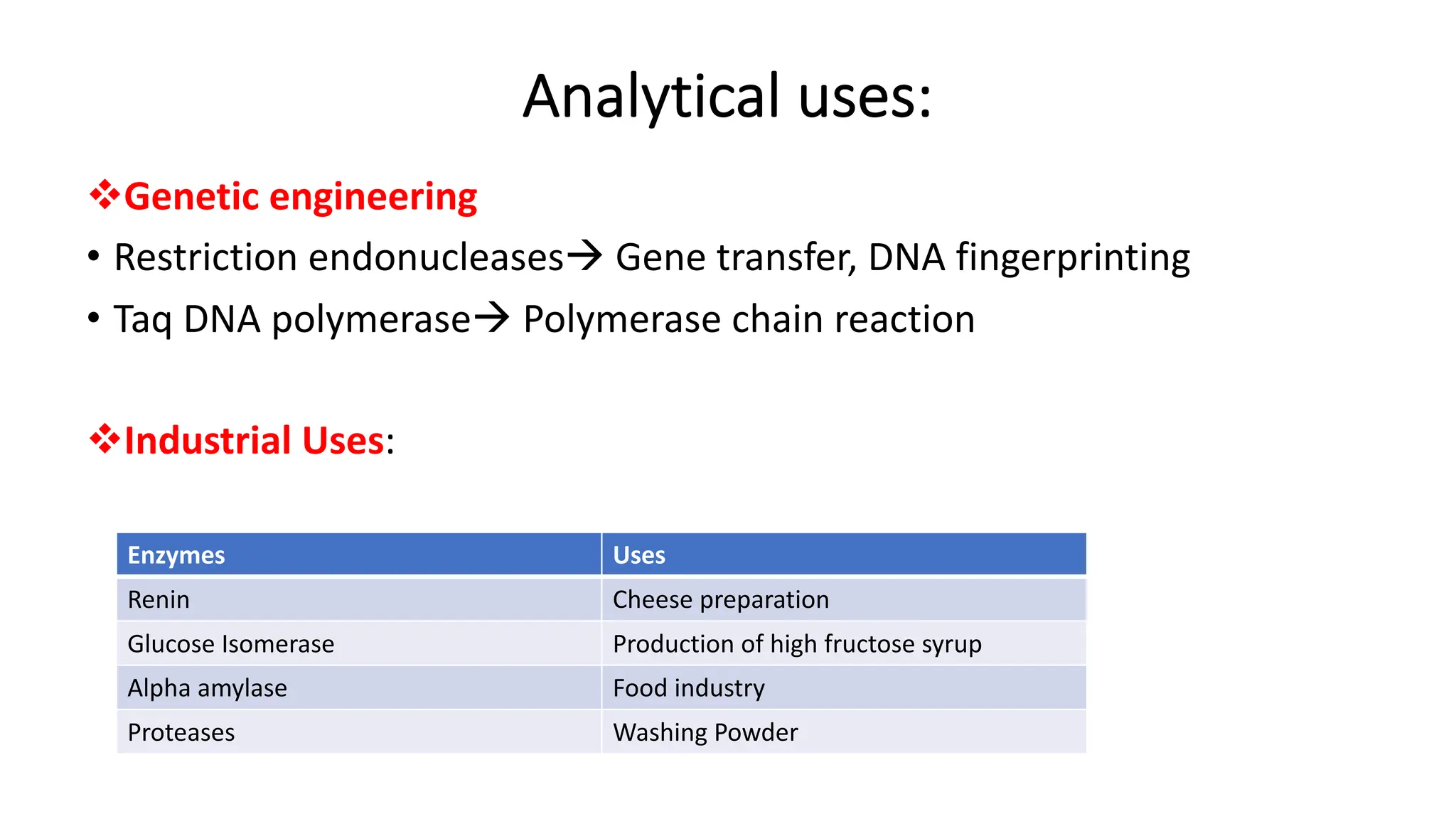
Many drugs act as enzyme inhibitors (e.g., statins inhibit HMG-CoA reductase).
👉 Do you want me to make a short exam-oriented description (2–3 lines), or a detailed one with examples for your MD Biochemistry
Introduction
Definition
Historical background (Pasteur, Buchner, Sumner, etc.)
Importance in life processes
2. General Characteristics of Enzymes
Catalytic power
Specificity
Efficiency
Reusability
Regulation
3. Structure of Enzymes
Protein nature
Apoenzyme and holoenzyme
Active site: features & models (lock and key, induced fit)
Cofactors, coenzymes, prosthetic groups
4. Classification of Enzymes
IUBMB classification – six main classes with examples
Enzyme Commission (EC) number system
5. Mechanism of Enzyme Action
Substrate binding
Transition state theory
Lowering activation energy
Catalytic strategies (acid–base catalysis, covalent catalysis, metal ion catalysis)
6. Kinetics of Enzymes
Michaelis–Menten equation
Lineweaver–Burk plot
Km and Vmax significance
Turnover number (Kcat)
Factors affecting enzyme activity: temperature, pH, substrate concentration, enzyme concentration, inhibitors
7. Enzyme Inhibition
Reversible inhibition (competitive, non-competitive, uncompetitive, mixed)
Irreversible inhibition (suicide inhibitors, poisons)
Clinical relevance (cyanide, organophosphates, drugs like statins, aspirin)
8. Regulation of Enzyme Activity
Allosteric regulation
Feedback inhibition
Covalent modification (phosphorylation, acetylation)
Isoenzymes
Induction and repression of