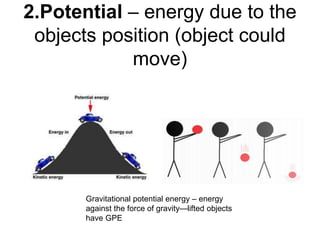
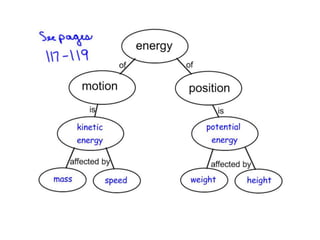
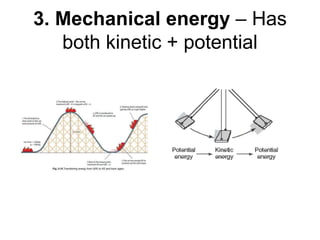
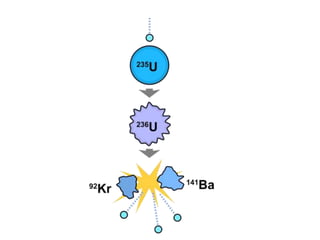
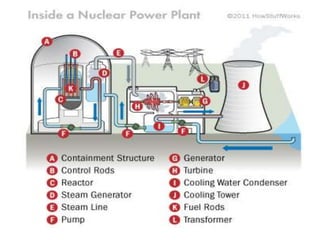
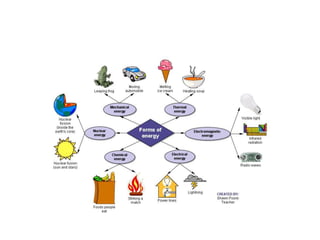
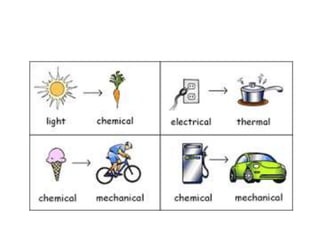
- Energy cannot be created or destroyed, but can be changed from one form to another through energy conversions. There are nine main types of energy including kinetic, potential, mechanical, thermal, chemical, electrical, sound, light, and nuclear energy.
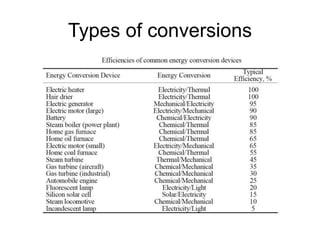
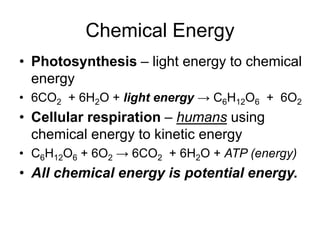
- Energy conversions allow useful energy to be extracted and support daily life through machines, though some energy is lost as thermal energy during conversions. Common conversions include light to chemical energy through photosynthesis and chemical to kinetic energy through cellular respiration.