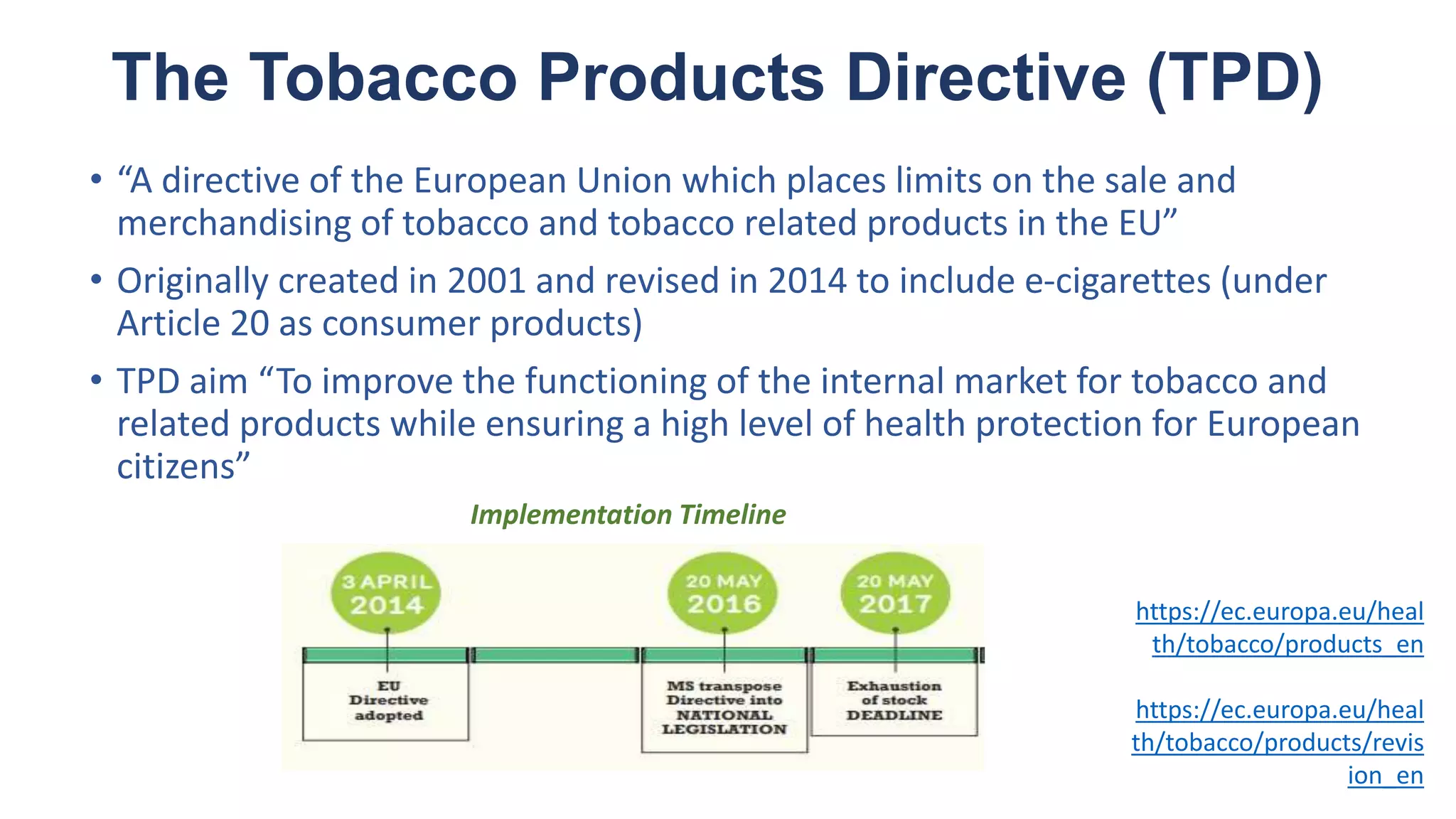
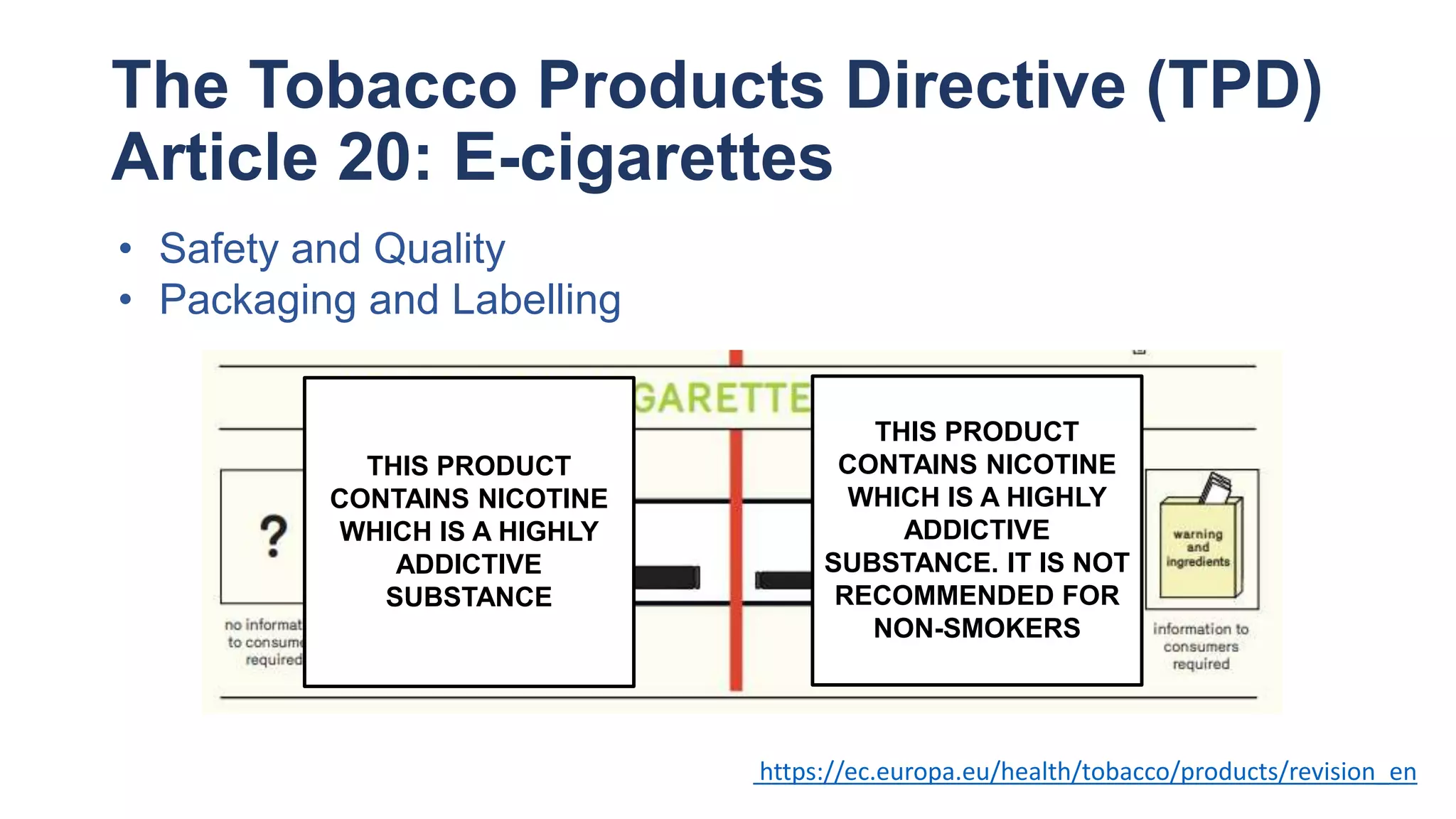
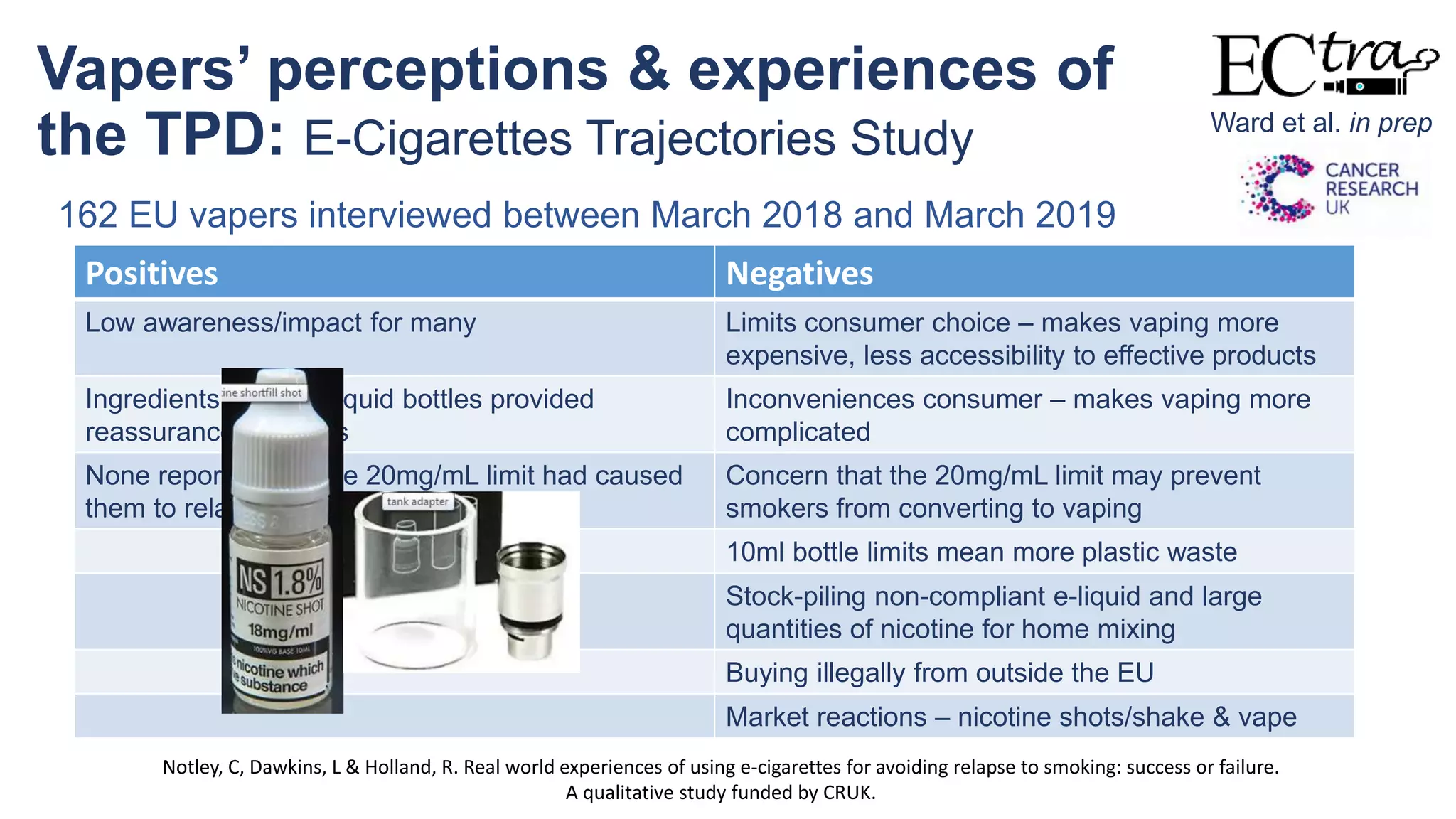
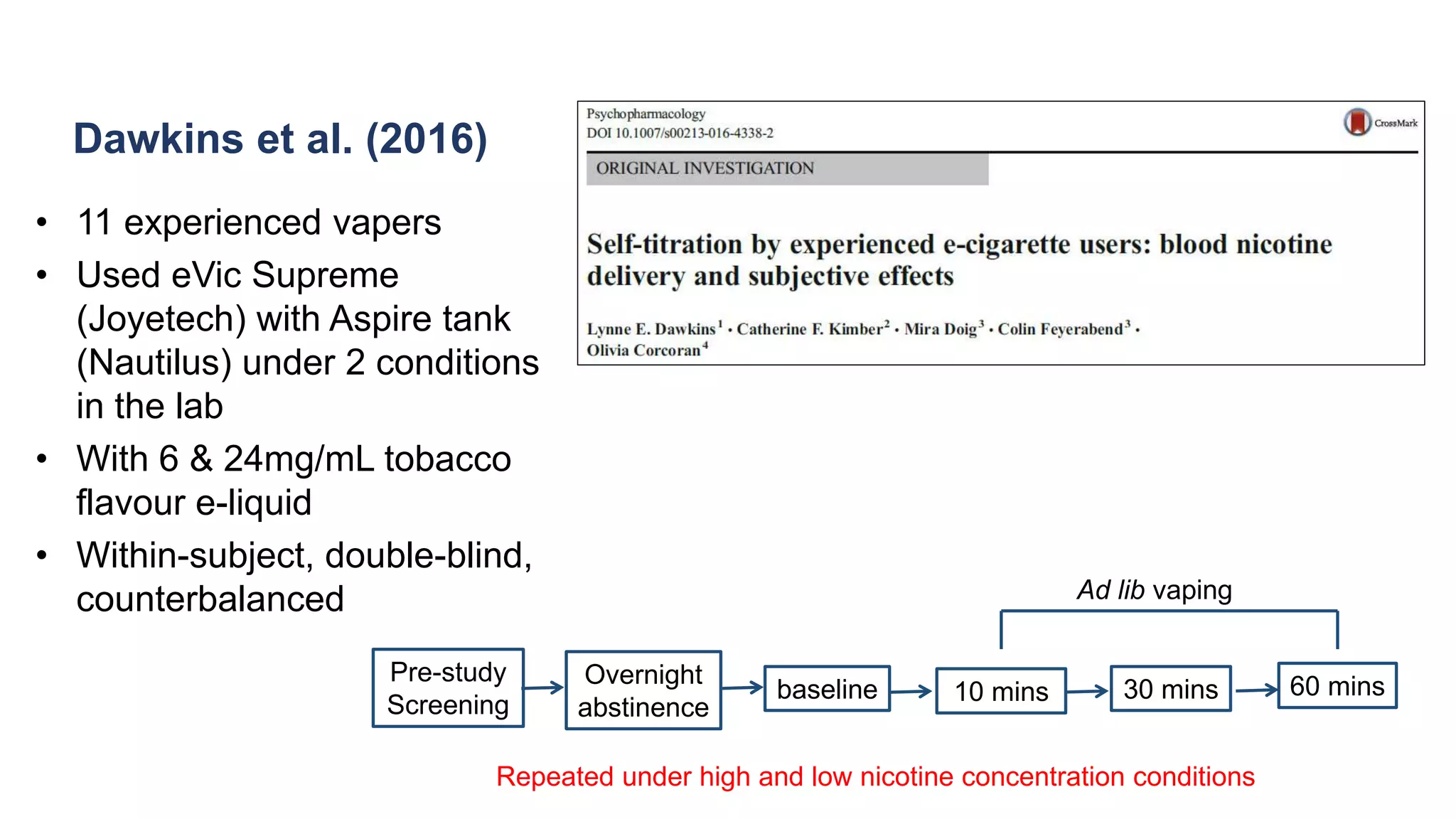
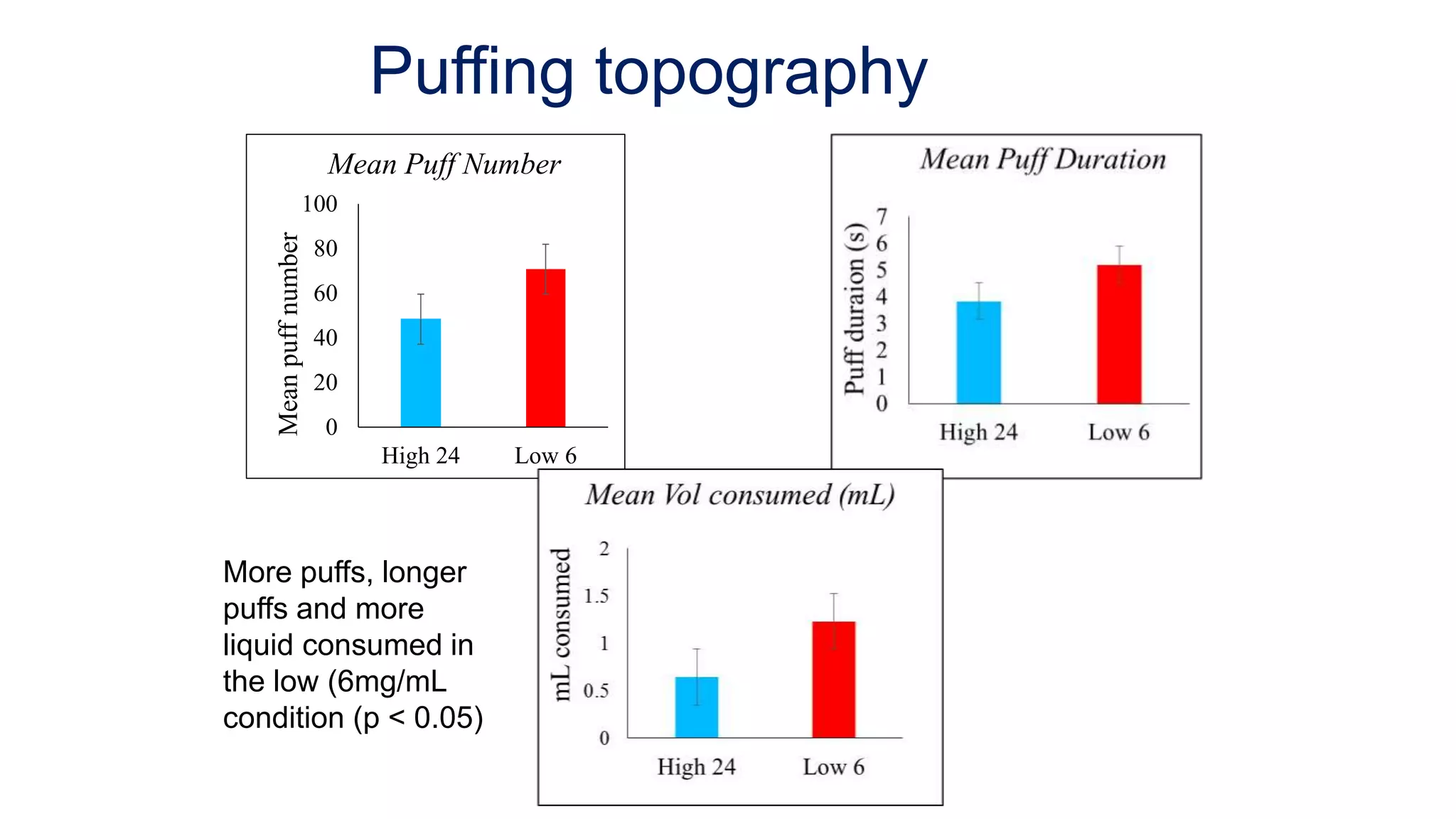
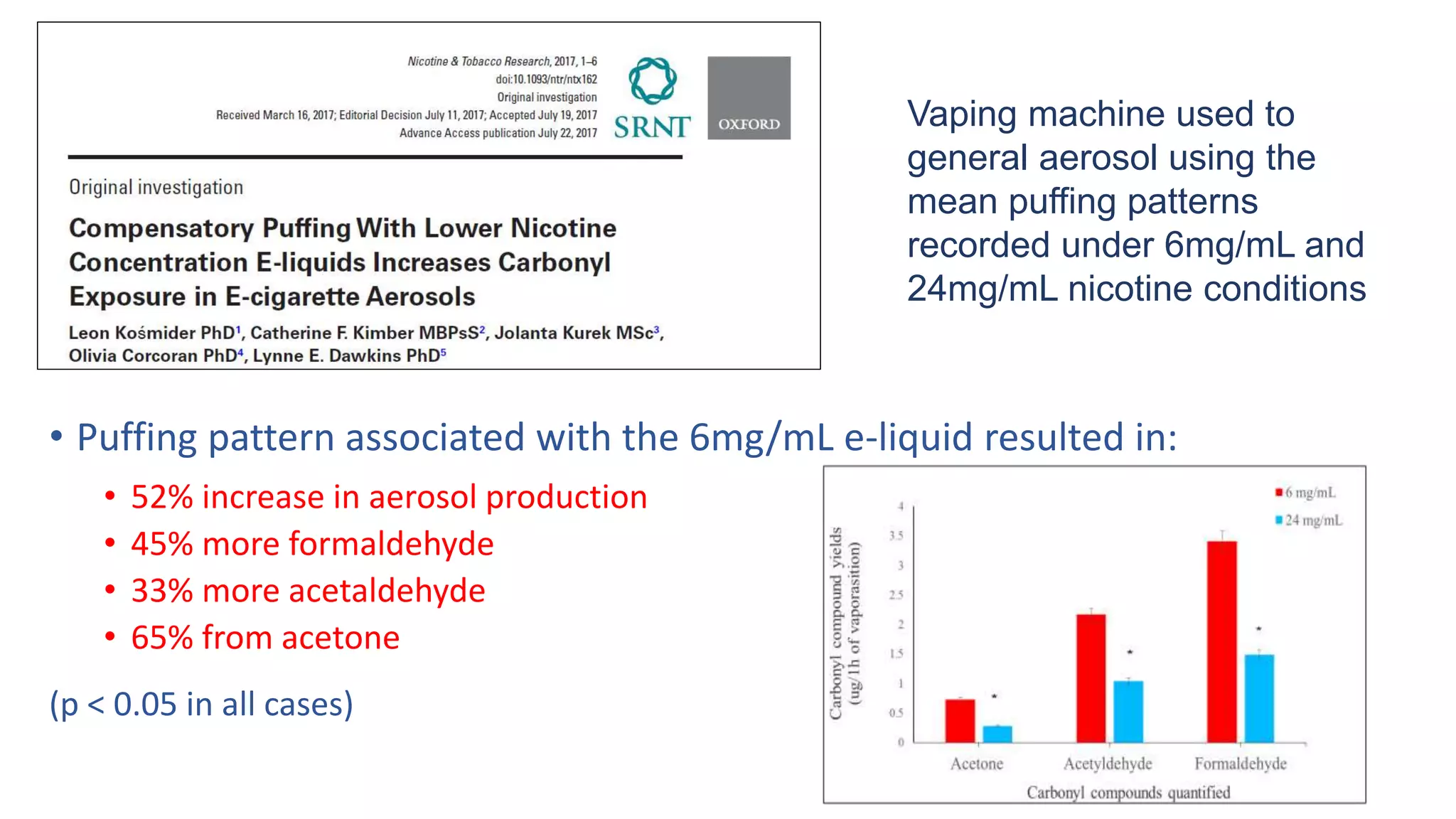
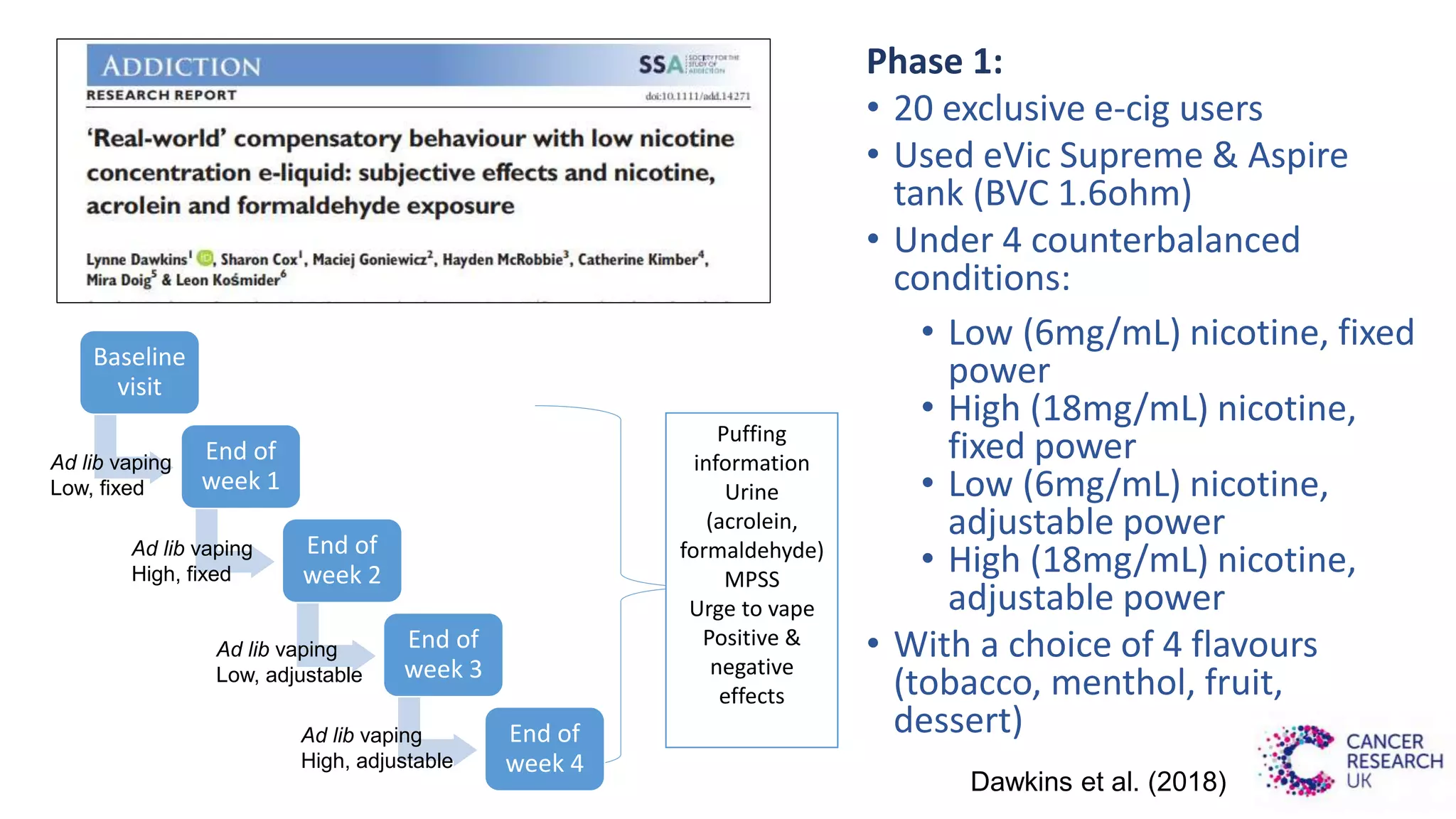
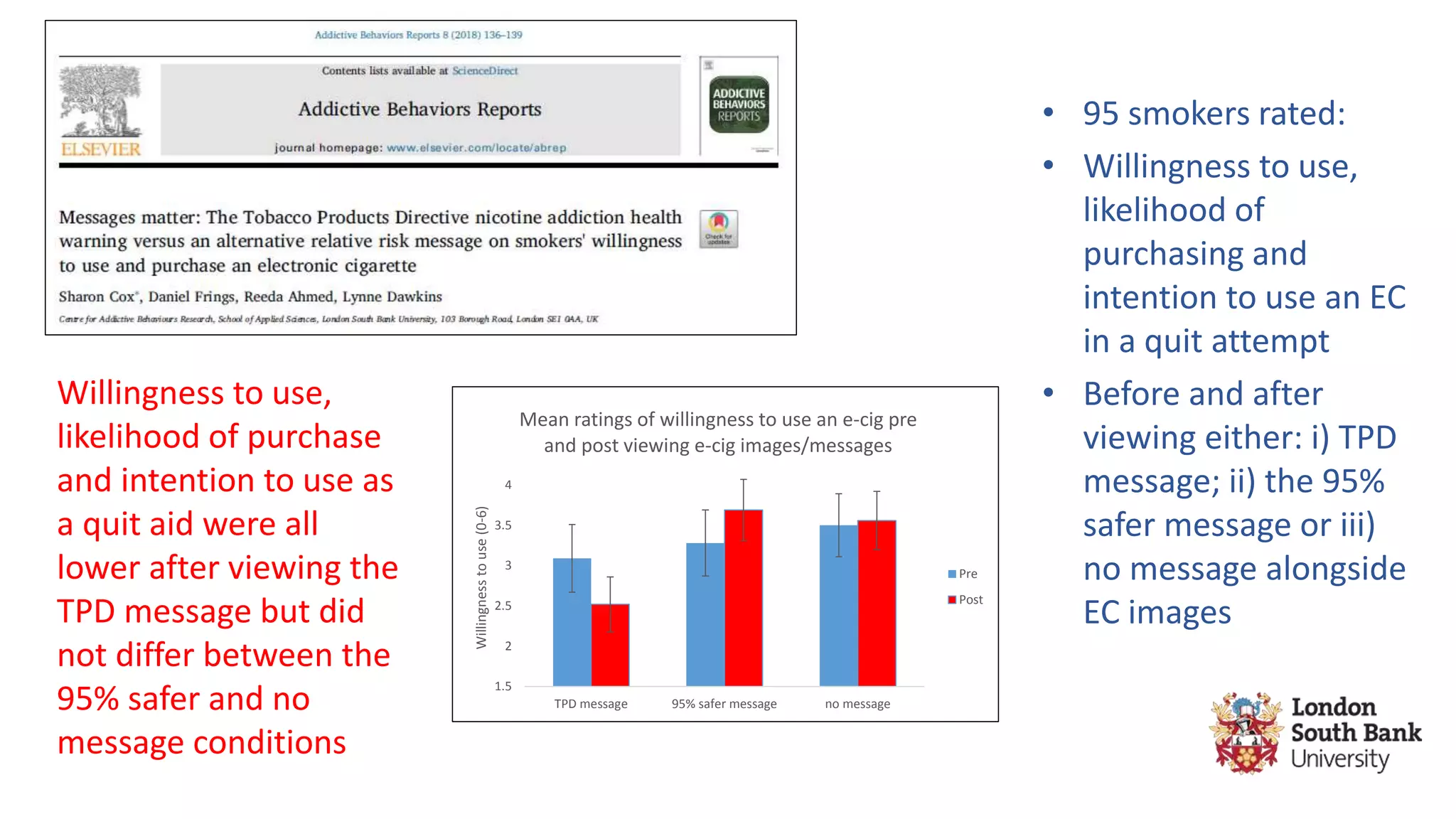
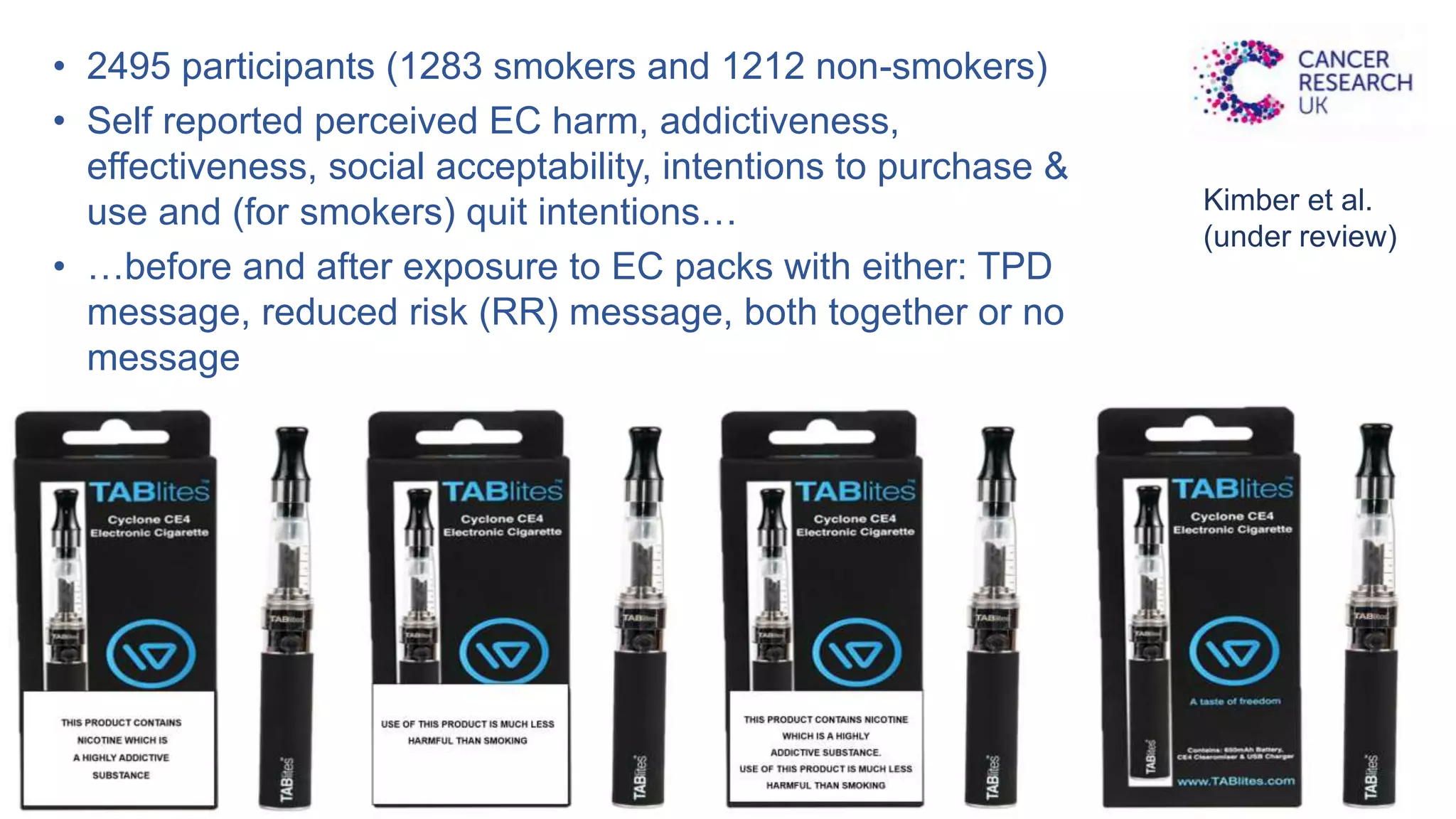
The document discusses the EU Tobacco Products Directive (TPD) implemented in 2014, which regulates e-cigarettes to enhance consumer safety and protect public health. Concerns arise regarding the pitfalls of the TPD, such as its potential to limit consumer choice, stifle innovation, and produce misleading perceptions about nicotine. The findings suggest that the current regulations may inadvertently perpetuate smoking by making vaping less accessible and less satisfying, raising questions about the effectiveness of public health policies in their intended goals.