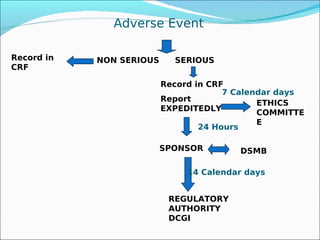
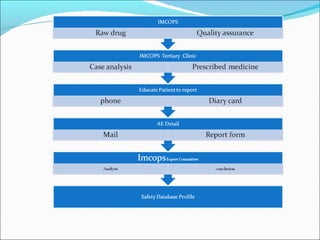
The document discusses adverse drug reactions and events, outlining key terms like adverse event, serious adverse event, and significance of adverse events. It provides examples of drugs that were withdrawn from the market like Rofecoxib due to increased risks of heart attacks and strokes found after approval. The document emphasizes the importance of reporting adverse events and reactions through proper forms and procedures, with serious events requiring expedited reporting to ethics committees, sponsors, and regulatory authorities within timelines. Proper implementation of adverse event reporting in Siddha medicine is highlighted.