National Center for Immunization & Respiratory Diseases
Rapid Diagnostic Tests for Bacterial Meningitis Pathogens: where we are now and what’s next.
Xin Wang Chief, Bacterial Meningitis Laboratory Director WHO Collaborating Center for Meningitis MVPDB/DBD/NCIRD/CDC Meningitis Research Foundation Conference Nov 1-3, 2021
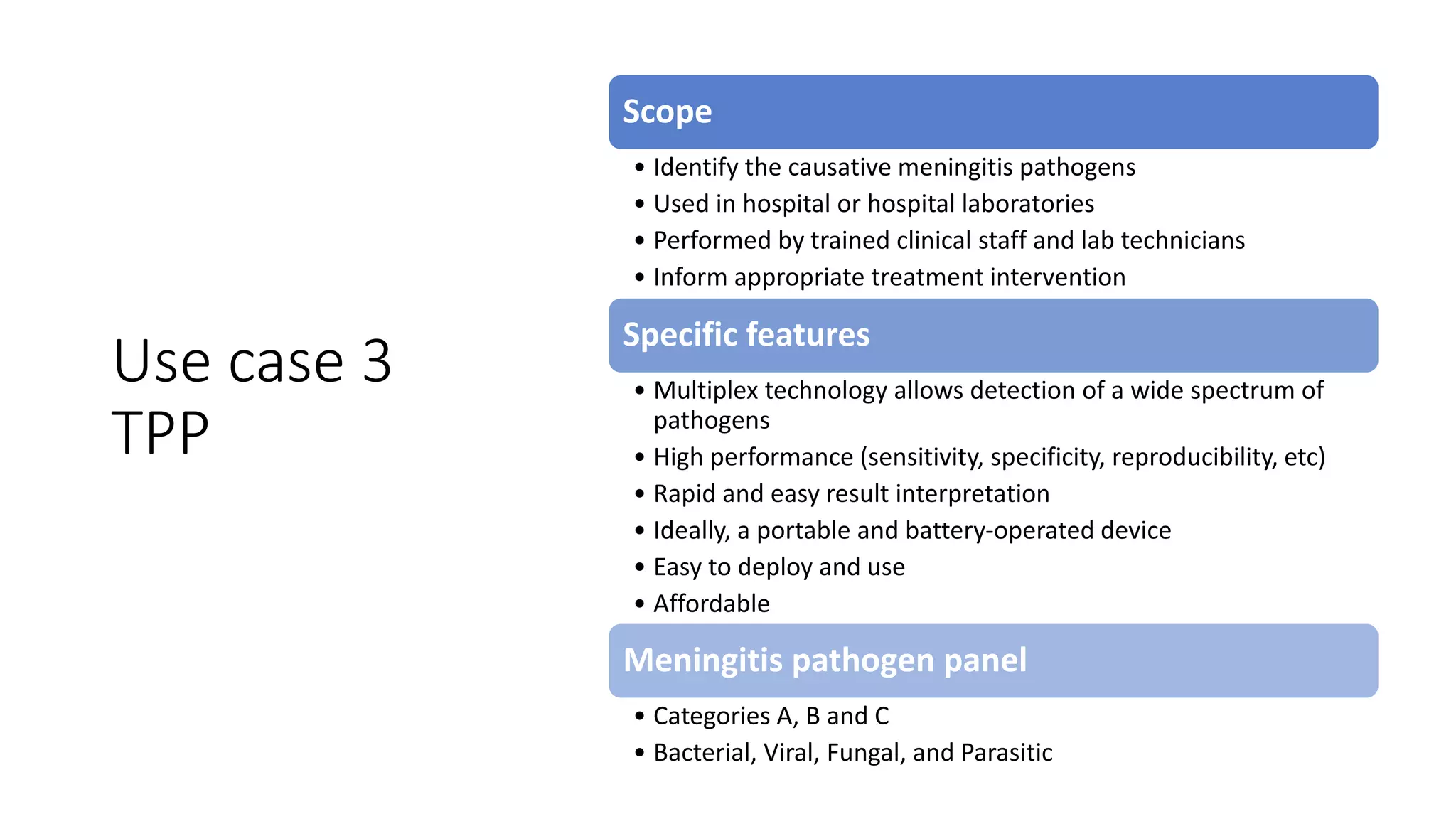
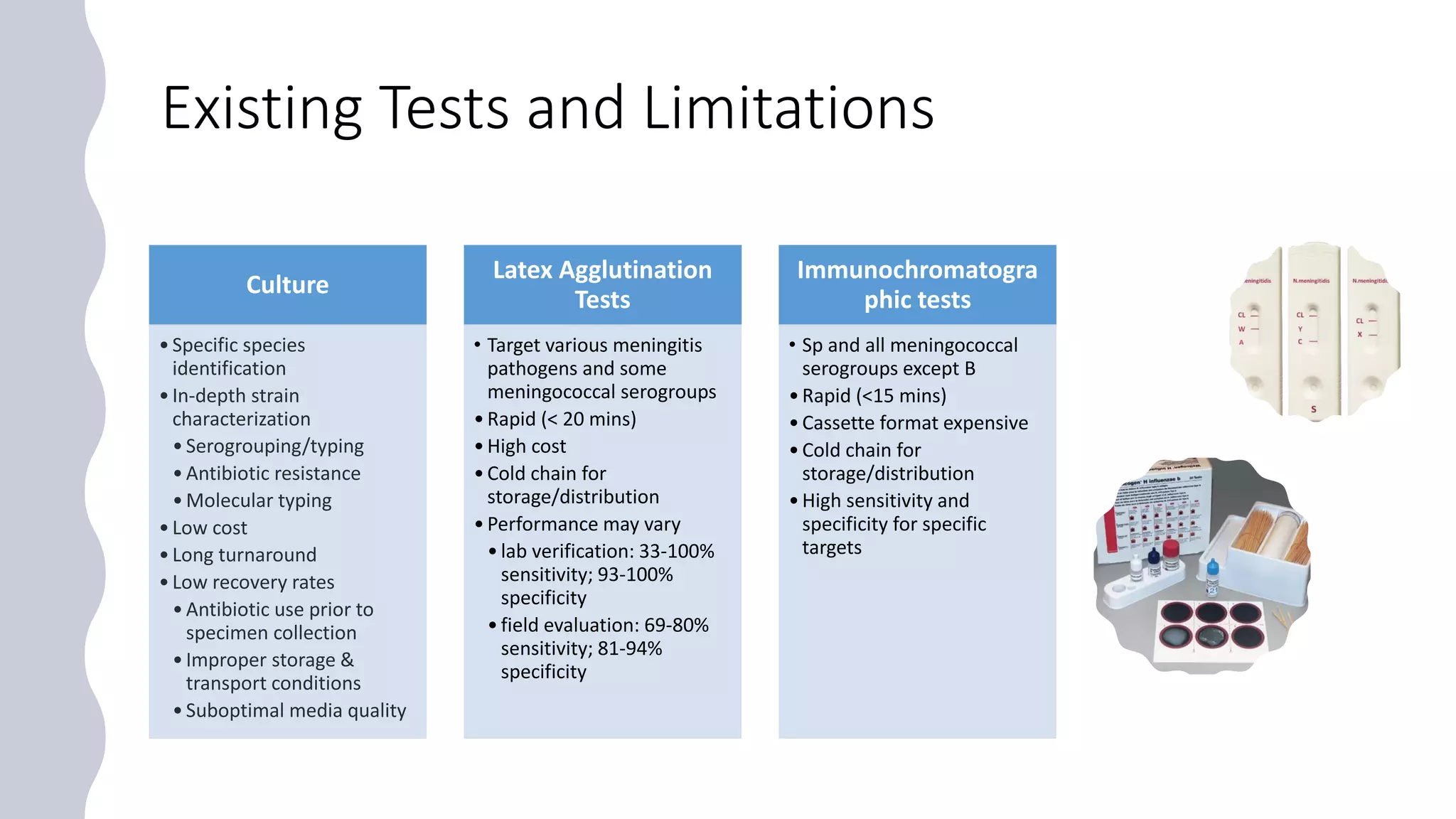
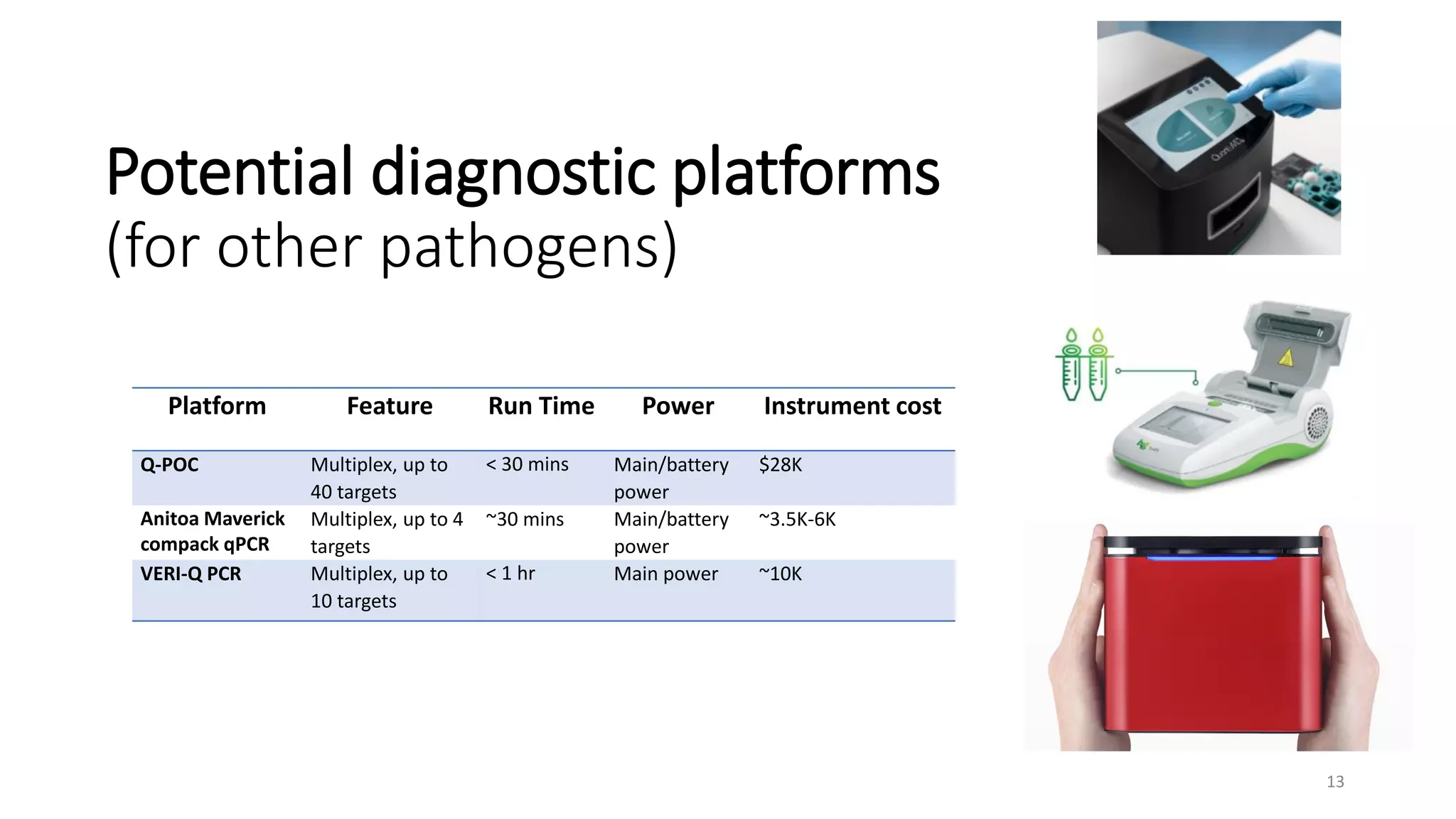
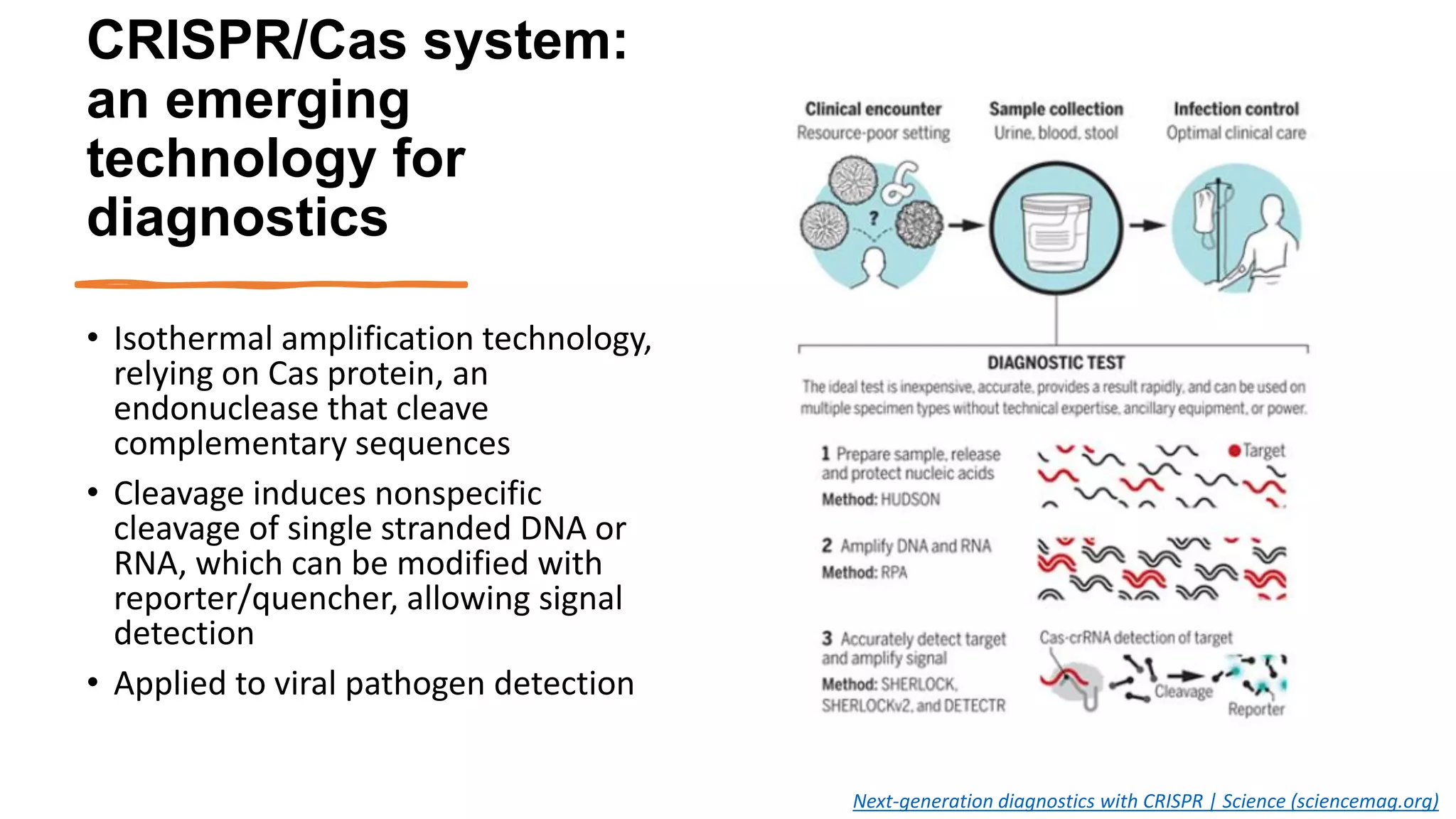
The document discusses the current state of rapid diagnostic tests for bacterial meningitis pathogens and outlines a vision for their future development and deployment. It describes existing tests and their limitations. Potential new platforms are identified that could meet targets outlined in a target product profile. Advanced technologies like sequencing and CRISPR/Cas are also discussed