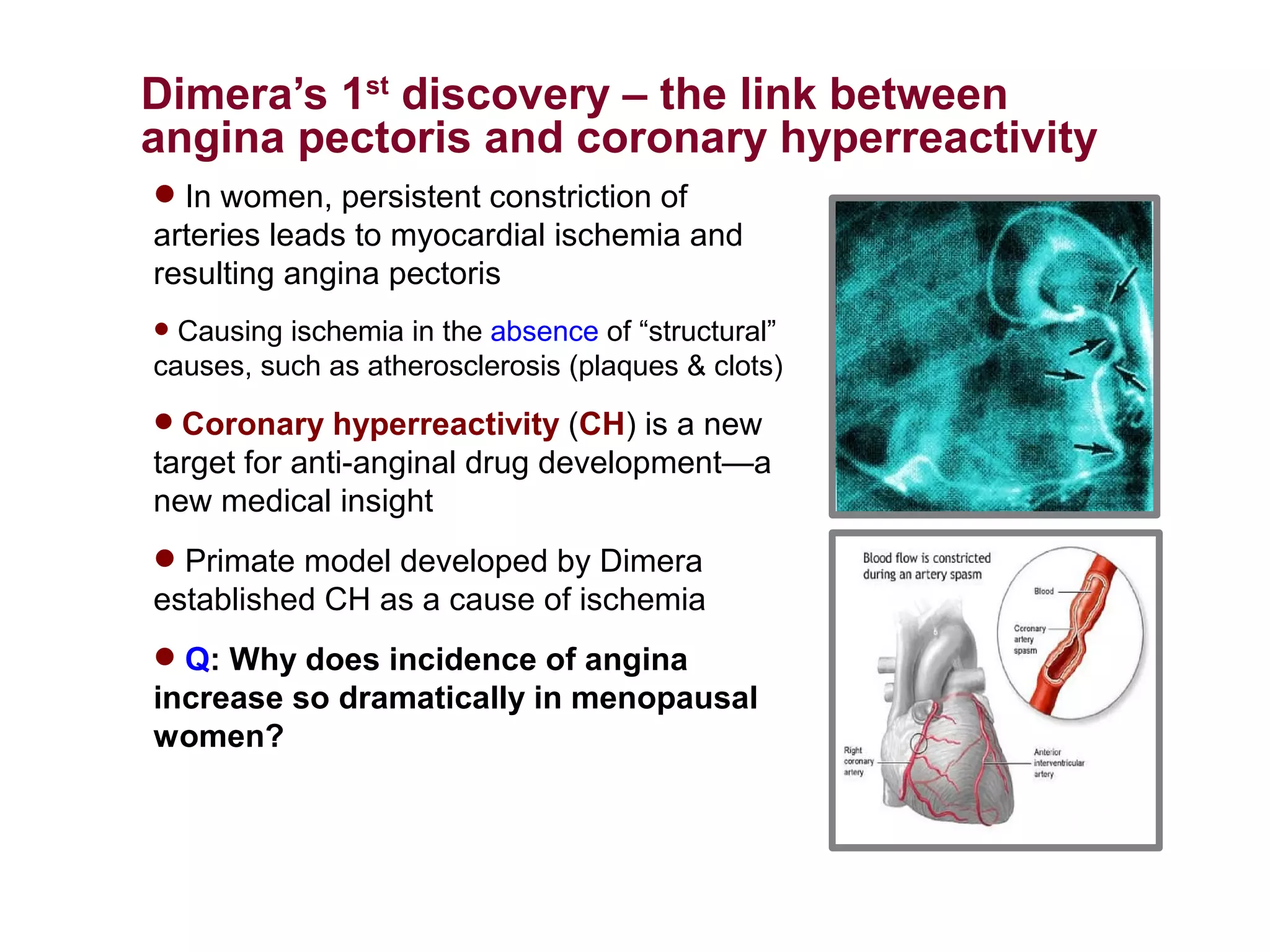
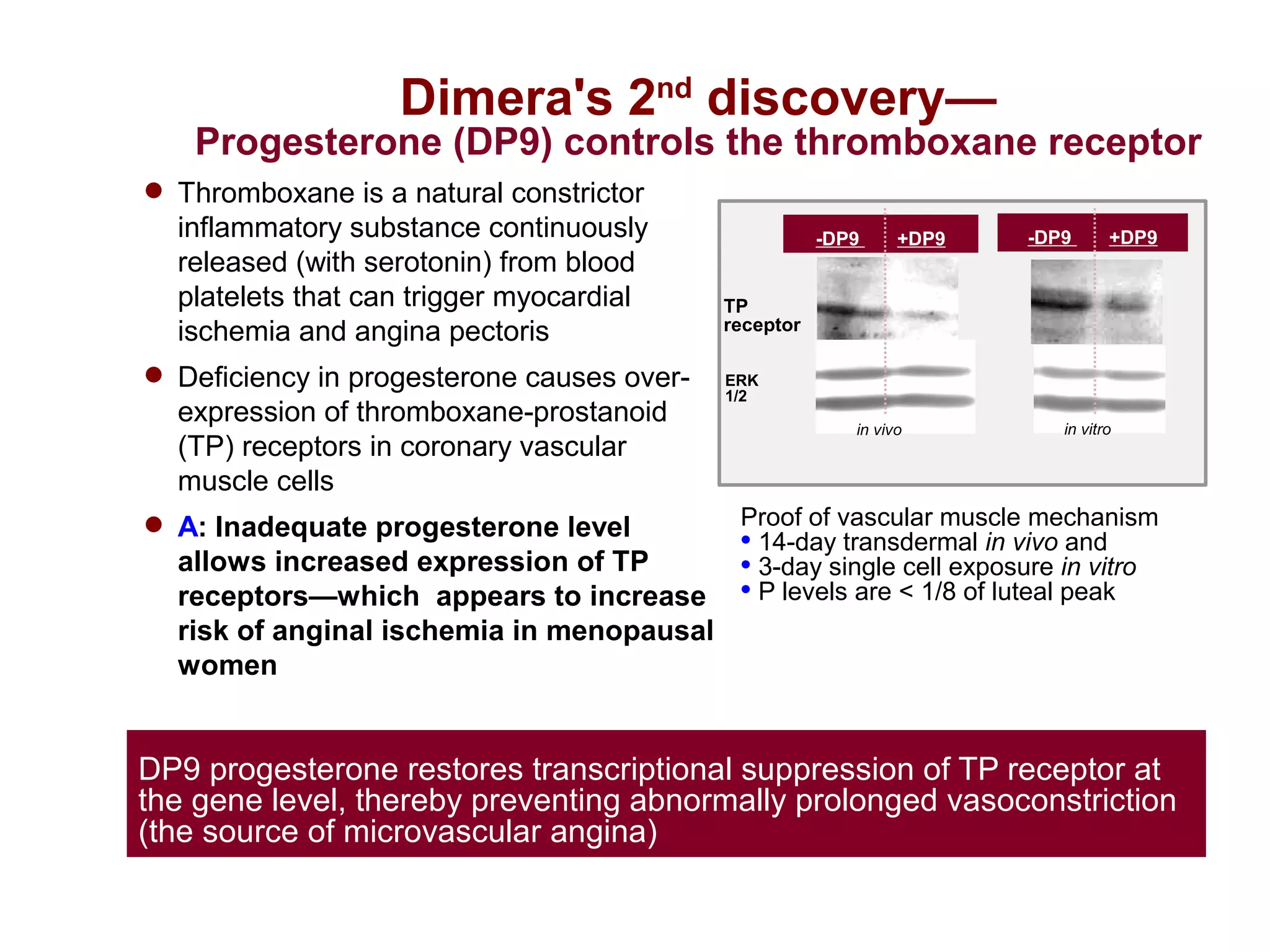
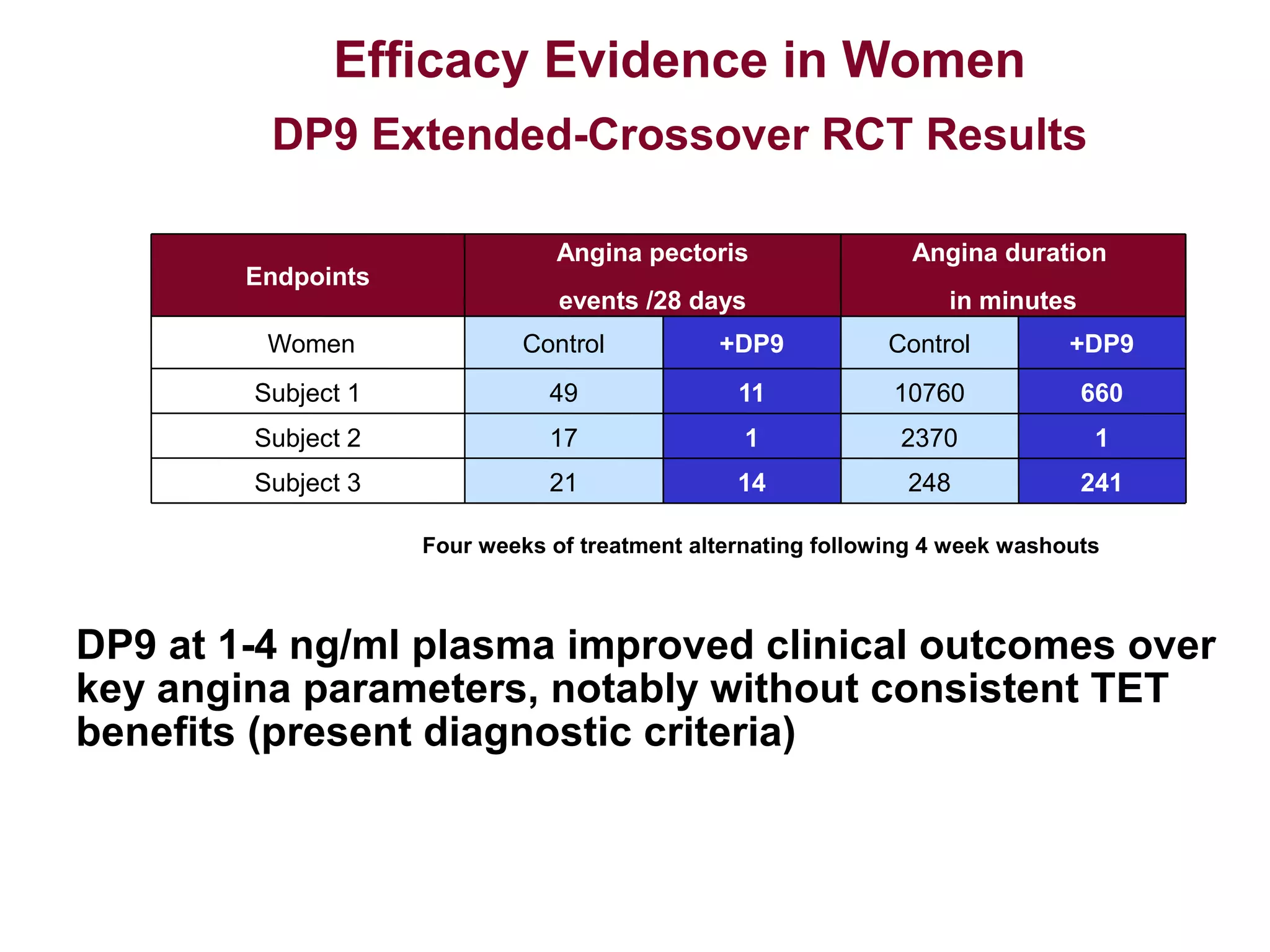
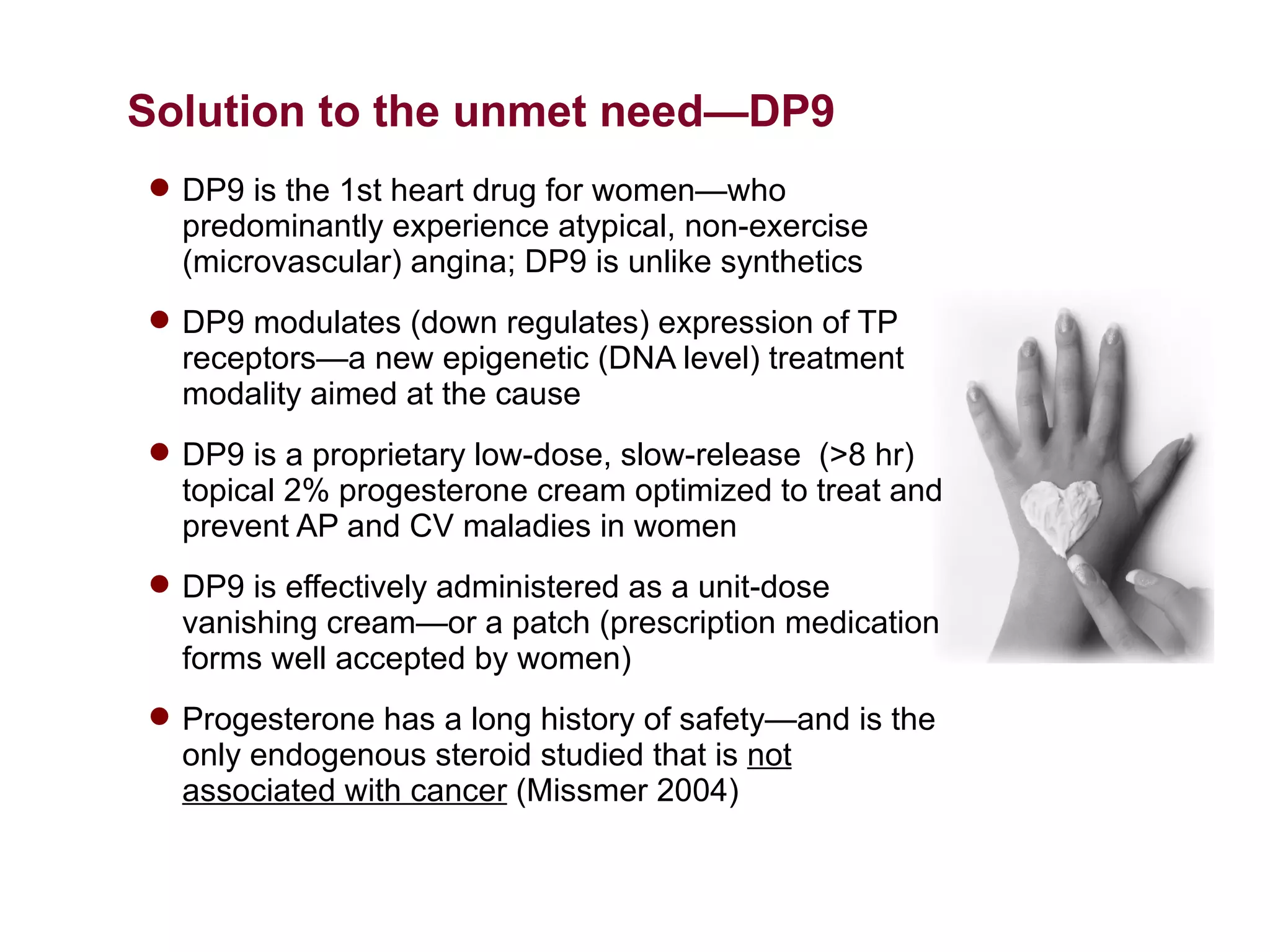
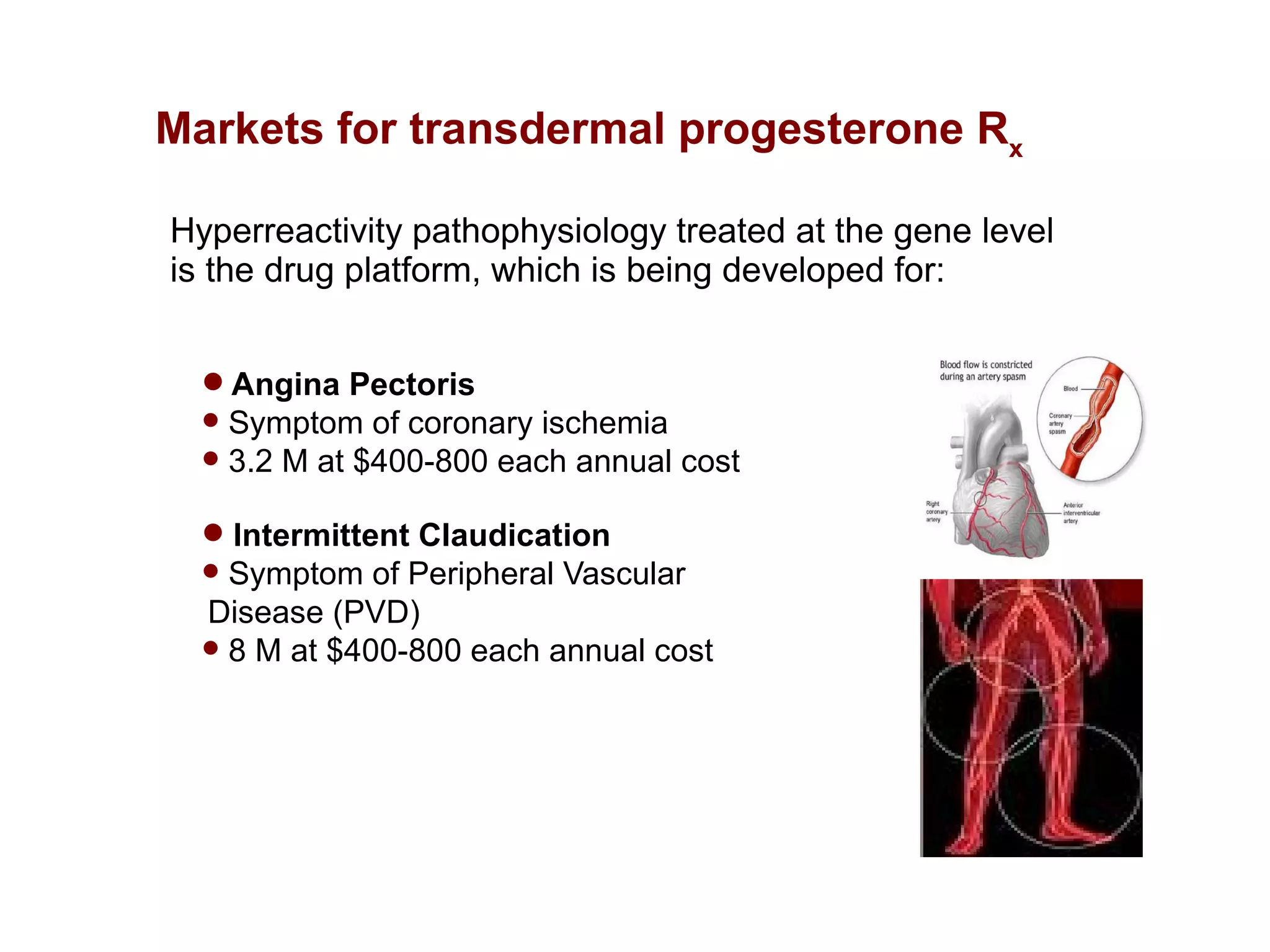
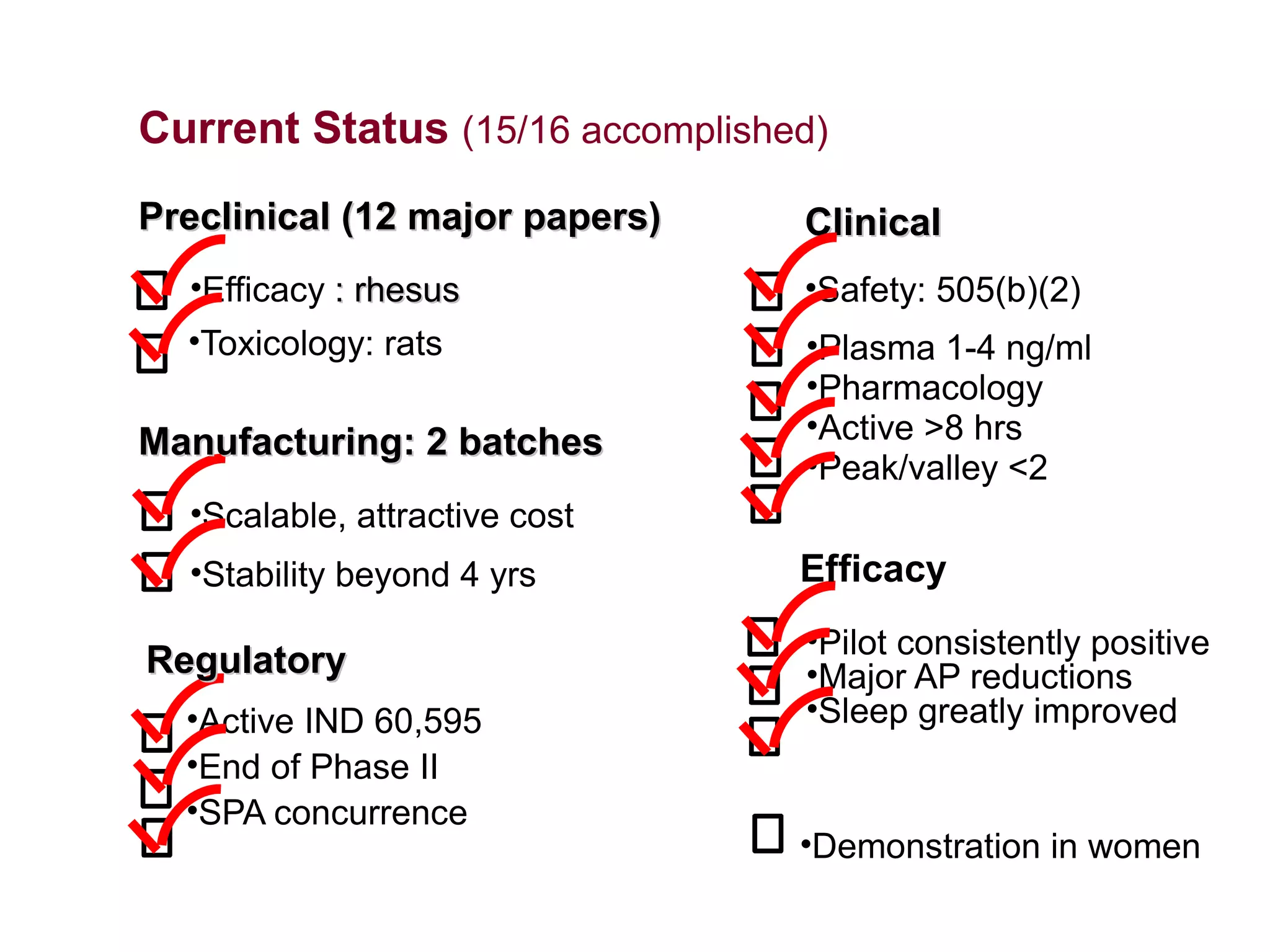
Dimera has developed a transdermal progesterone cream called DP9 to treat angina pectoris in women. Over 500,000 women die annually from cardiovascular disease, yet current drugs do not effectively treat the symptoms experienced by many menopausal women. DP9 aims to downregulate thromboxane receptor expression at the gene level to prevent abnormal vasoconstriction. Clinical trials show DP9 significantly reduces angina events and duration of symptoms. With over 3 million women suffering from treatment-resistant angina, DP9 has the potential to become a blockbuster drug as the first FDA-approved treatment tailored to women's heart health needs.